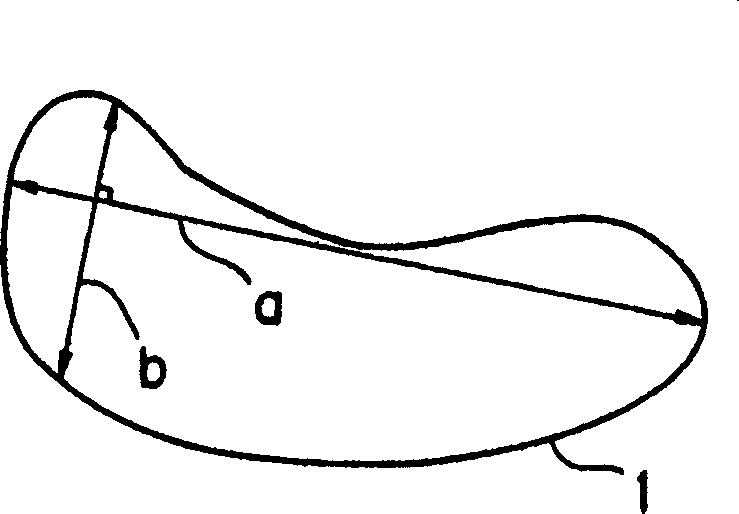
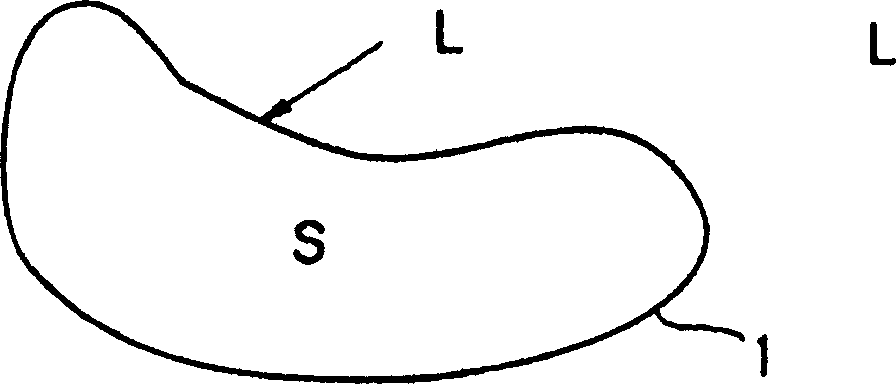
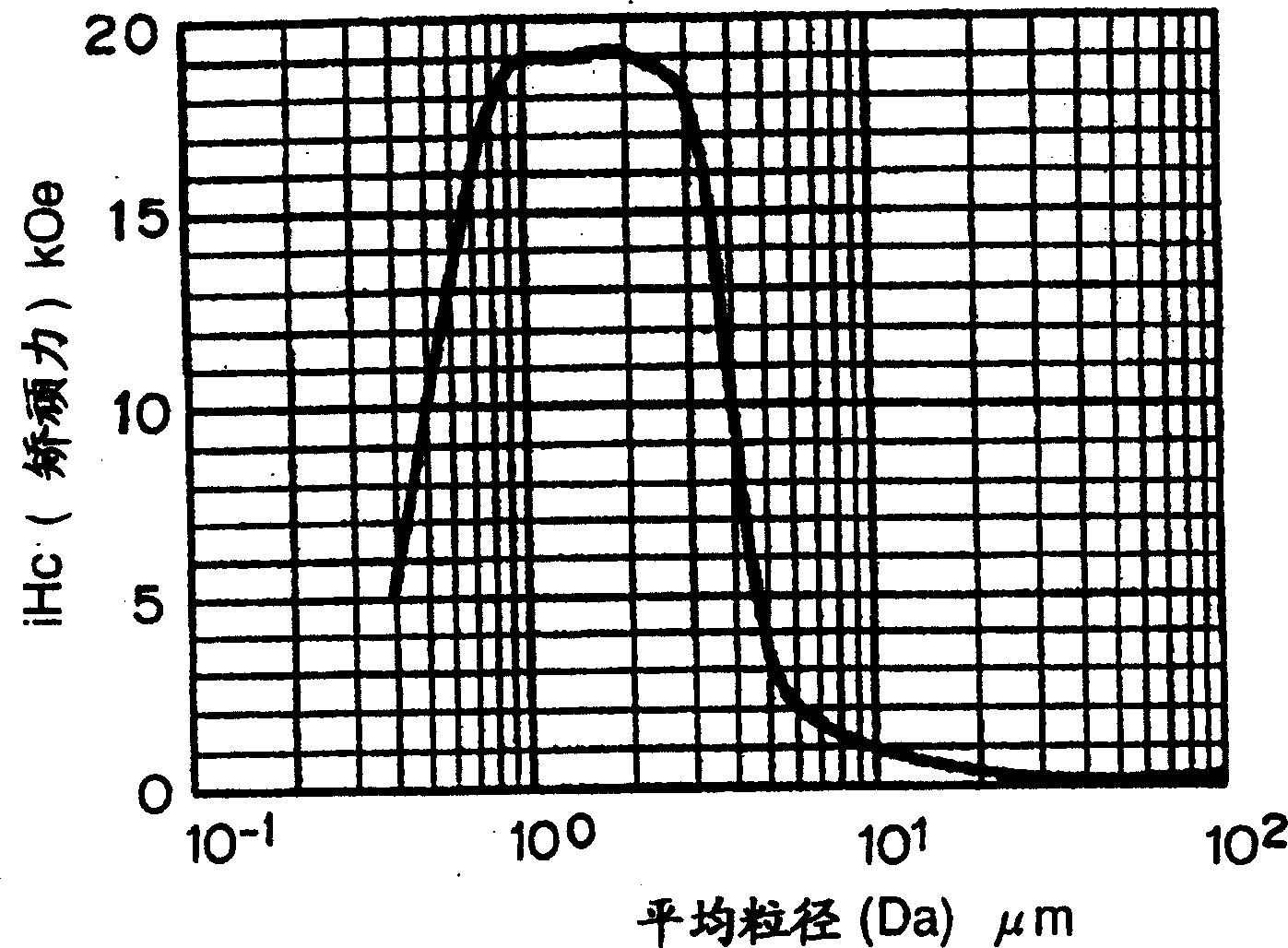
Process for producing Sm-Fe-N alloy powder
A technology of alloy powder and manufacturing method, applied in the direction of chemical instruments and methods, nitrogen compounds, non-metallic elements, etc., which can solve the problems of poor alignment and orientation, low filling rate of bonded magnets, and limited magnet powder ratio, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] 1. Precipitation reaction
[0098] Add 30 liters of pure water to the reaction tank, which added 520g of 97% H 2 SO 4 , dissolved 484.8 g of Sm 2 o 3 , and then add 25% ammonia water to roughly adjust the pH value to neutral. Add 5200g of FeSO to this aqueous solution 4 ·7H 2 O, make it dissolve completely and become a metal solution.
[0099] Add 12 liters of pure water in another tank, mix the 25% ammoniacal liquor of 2524g of ammonium bicarbonate and 1738g in water, be modulated into ammonia carbonate solution. While stirring the reaction tank, slowly add the above-mentioned ammonium carbonate solution to the metal solution, and then add ammonia water, so that the final pH value after all the addition is 8.0±0.5. After the agitation stops, the solution is allowed to stand in the tank, allowing the product to settle to the bottom of the container. When a part of the precipitate obtained at this time was taken out and observed under a microscope, all the partic...
Embodiment 2
[0115] 1. Co-precipitation
[0116] Weigh the nitric acid of 513.4g to be enough for the hydrate Sm(NO 3 ) 3 ·6H 2 O and 3432.3g of iron nitrate 9 water hydrate Fe(NO 3 ) 3 9H 2 O, both are dropped into 10 liters of ion-exchanged water together, and stir simultaneously. After confirming to dissolve completely, continue to stir, add the urea (NH2) of 2992.5g simultaneously 2 ) 2 CO, then, while continuing to stir, the liquid temperature was raised to 80 °C. At this time, urea is decomposed into ammonia gas and carbon dioxide gas due to the addition of water, and the metal-containing material is precipitated due to uniform reaction.
[0117] 2. Filter and wash
[0118] The resultant was placed on filter paper, and particle-exchanged water was poured over the filter paper to pass through the filter paper. This operation is not stopped until the specific resistance of the filtrate is lower than 50μs / m. The washed product cake was dried in a dryer at 80°C.
[0119] 3. C...
Embodiment 3
[0132] The average particle size of 1.5 microns, the iron oxide (Fe 2 o 3 ) powder 135.7g, average particle size 1.0 micron, samarium oxide powder (Sm 2 o 3 ) 34.9g, mixed with water with a ball mill and pulverized for 2 hours. The iron oxide powder and samarium oxide powder used in this example are the same materials as in Example 1. After the obtained slurry is dehydrated, the solid matter is separated, and the dried solid matter is crushed with a sample pulverizer to obtain a mixed powder. The obtained mixed powder was put into a pan made of mild steel, and then put into a furnace for preliminary reduction in a hydrogen stream at 600°C. During the reduction process, the flow rate of hydrogen was 2 liters / minute, and the duration was 5 hours.
[0133] The oxygen analysis result of the reduced powder showed that the oxygen element removal rate of the iron oxide component was 89.5%. However, under such conditions, samarium oxide was not reduced by hydrogen. Add 44.5 g o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Curie point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com