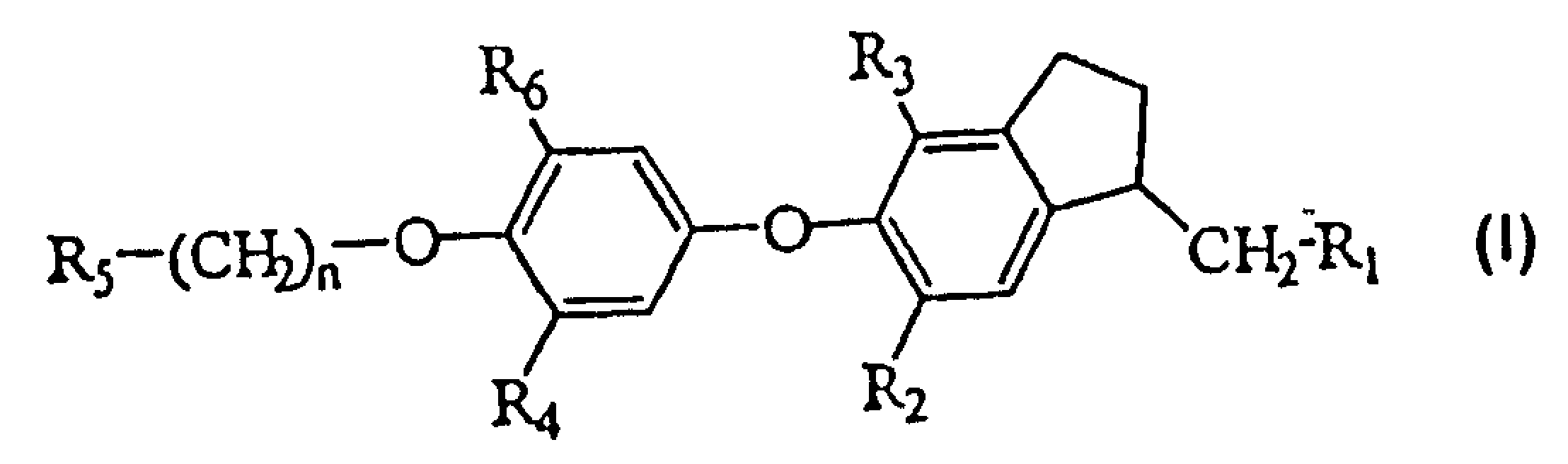
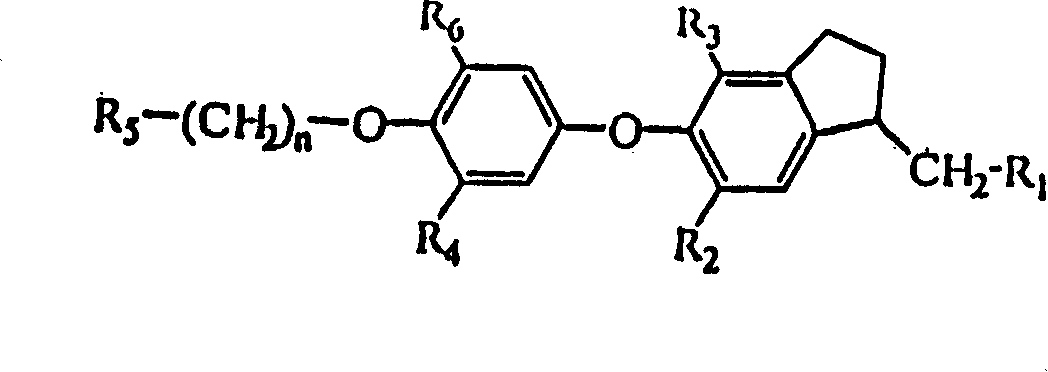
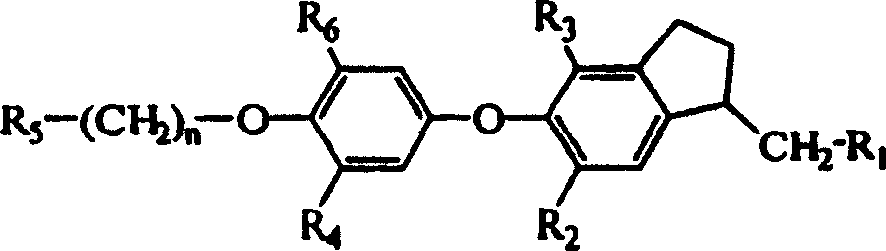
Novel thyroid receptor ligands
一种甲氧基、化合物的技术,应用在新化合物领域,能够解决效能和耐受性未知等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] {4,6-Dibromo-5-[3-isopropyl-4-(naphthyl-2-methoxy)phenoxy]indanyl-1}acetic acid
[0080] (a) Add sodium acetate (7.0 g, 83 mmol) to a mixture of 5-hydroxy-1-indanone (5.6 g, 38 mmol), acetic acid (260 ml) and 4-5 drops of water, then add dropwise Bromine (13.3 g, 83 mmol) in acetic acid (60 mL). The reaction mixture was stirred at room temperature for 18 hours, and the precipitate was filtered and dried. 8.4 g (72%) of 4,6-dibromo-5-hydroxy-1-indanone were obtained as a solid, which was used directly in the next experiment without further purification.
[0081] (b) In a mixture containing bis-(3-isopropyl-4-methoxyphenyl)iodonium tetrafluoroborate (6.25 g, 12.2 mmol), bronze (1.02 g) and dichloromethane (25 mL ) in a stirred suspension system containing 4,6-dibromo-5-hydroxyl-1-indanone (2.50 g, 8.17 mmol) and triethylamine (1.00 g, 8.99 mmol) in nitrogen at room temperature Dichloromethane solution (25 mL). The reaction mixture was stirred for 48 hours in the dark ...
Embodiment 2
[0087] {4,6-Dibromo-5-[4-(4-fluorobenzyloxy)-3-isopropylphenoxy]indanyl-1}acetic acid
[0088] 4-Fluorobenzyl bromide (15 mg, 0.080 mmol) and ethyl[4,6-dibromo-5-(3-isopropyl-4-hydroxyphenoxy)indanyl-1]acetate (20 mg, 0.039 mmol) was coupled and then worked up according to the procedure described in Example 1. Obtained 5.3 mg (23%) of {4,6-dibromo-5-[4-(4-fluorobenzyloxy)-3-isopropylphenoxy]indanyl-1}acetic acid. LC-MS (ES) m / z 591 (M-1).
Embodiment 3
[0090] {4,6-Dibromo-5-[3-isopropyl-4-(5-methylisoxazolyl-3-methoxy)phenoxy]indanyl-1}acetic acid ethyl[4 , 6-dibromo-5-(3-isopropyl-4-hydroxyphenoxy)indanyl-1] acetate (20 mg, 0.039 mmol), potassium carbonate (11 mg, 0.080 mmol) The mixture with acetonitrile (0.75 mL) was stirred at room temperature for 30 minutes. 3-Chloromethyl-5-methylisoxazole (10.5 mg, 0.080 mmol) and a catalytic amount of potassium iodide in acetonitrile (0.25 mL) were added, and the reaction mixture was stirred at 80°C. After 16 hours, the reaction mixture was purified on a short column (SPE-silica, 1 g / 6 mL, n-heptane / ethyl acetate 65:35), the resulting filtrate was concentrated, the residue and THF (0.50 mL) and Lithium hydroxide (0.5 mL, 1N) was stirred at room temperature for 16 hours. The reaction mixture was filtered through an SCX-column (strong cation exchanger: silane benzenesulfonate, 1 g / 3 ml, eluting with methanol), and the filtrate was concentrated. The filter residue was dissolved with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com