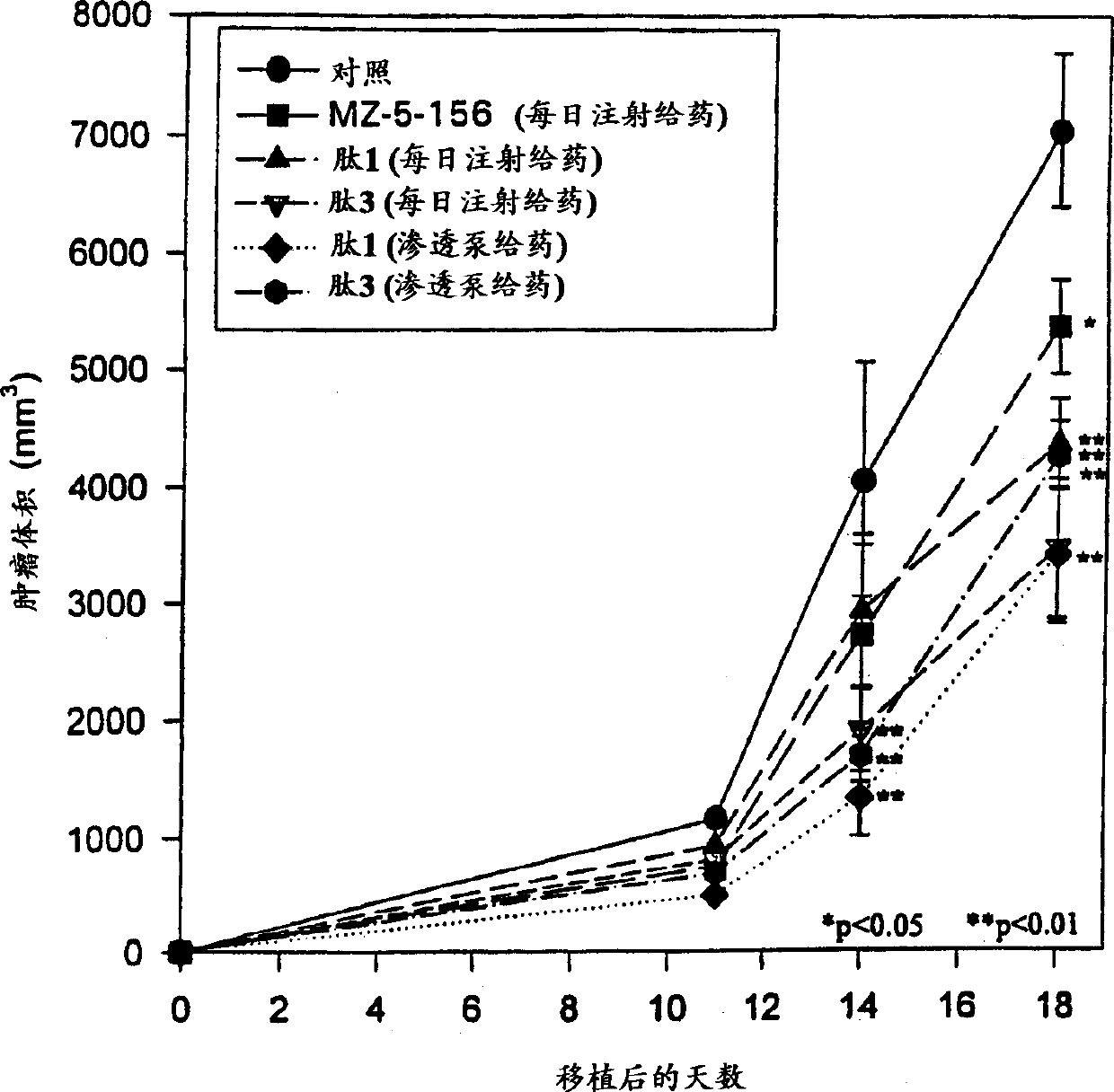
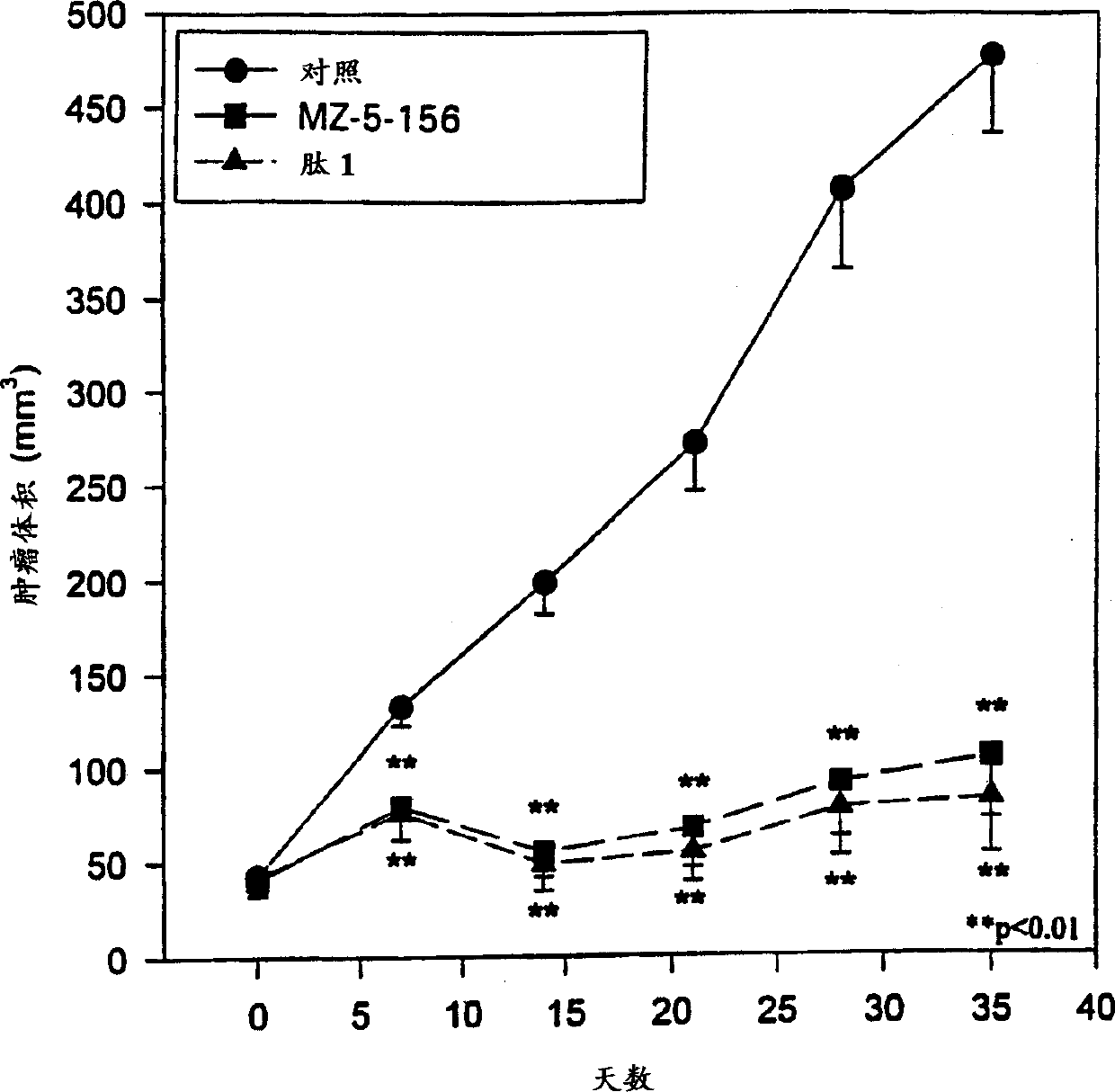
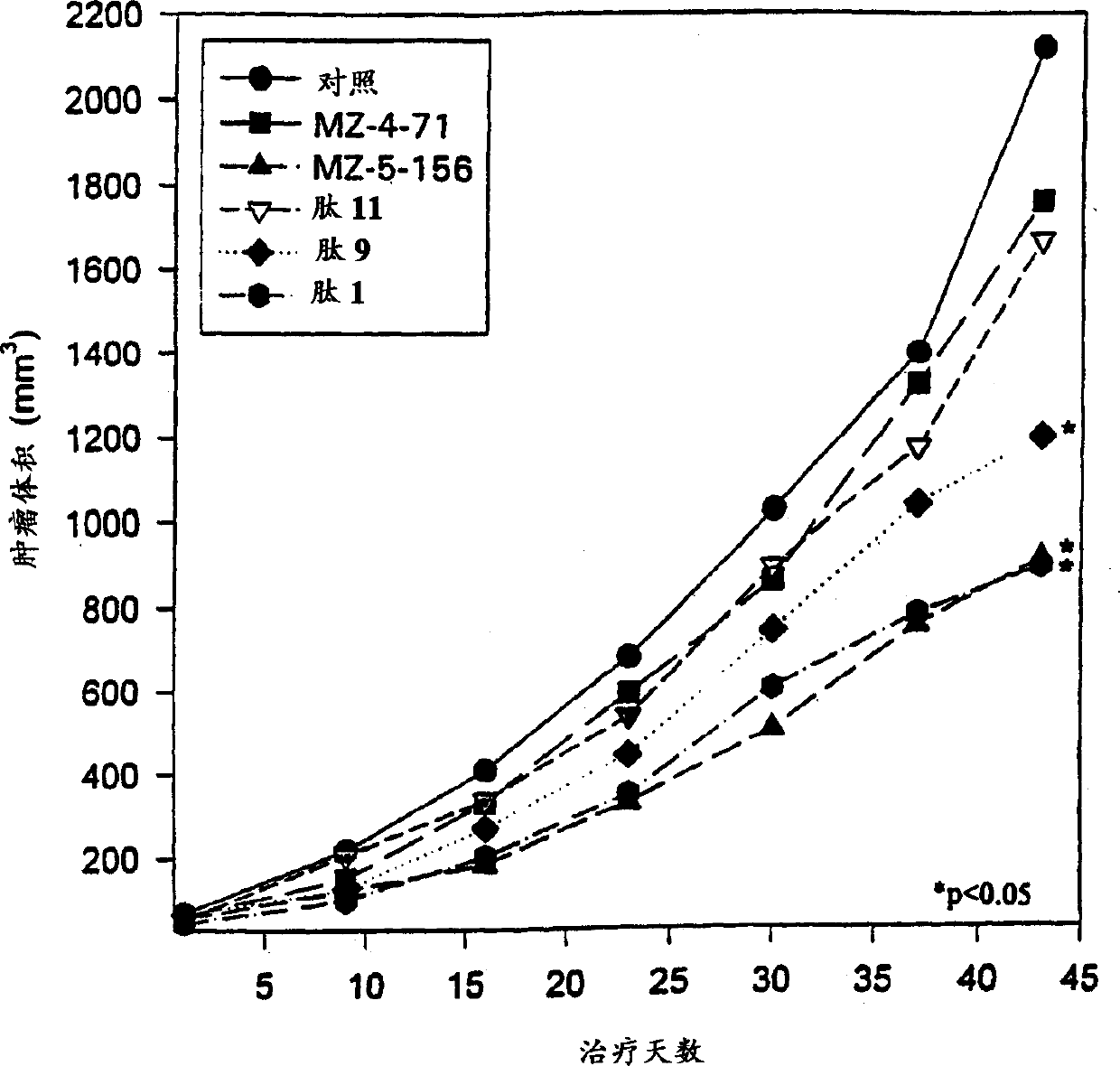
Antagonistic analogs in GH-RH inhibiting IGF-I and -II
A compound, R12 technology, applied in the field of new synthetic peptides, government-funded, can solve the problem of inferior antagonist properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0125] Example IPhAc 0 -Tyr 1 -D-Arg 2 -Asp 3 -Ala 4 -Ile 5 -Phe(pCl) 6 -Thr 7 -Asn 8 -Arg 9 -Tyr 10 -Arg 11 -Lys 12 -Val 13 -Leu 14 -Abu 15 -Gln 16 -Leu 17 -Ser 18 -Ala 19 -Arg 20 -Lys 21 -Leu 22 -Leu 23 -Gln 24 -Asp 25 -Ile 26 -Nle 27 -D-Arg 28 -Har 29 -NH 2 (peptide 1){[PhAc 0 , D-Arg 2 , Phe(pCl) 6 , Arg 9 , Abu 15 , Nle 27, D-Arg 28 , Har 29 ]hGH-RH(1-29)NH 2}
[0126] The synthesis of this peptide was carried out in a step-by-step manner using a manually operated solid-phase peptide synthesis device. Briefly, following the scheme described in Table 1 with CH 2 Cl 2 Para-methylbenzhydrylamine (MBHA) resin (Bachem, California) (720 mg, 0.50 mmole) was neutralized with formulated 5% DIEA and washed. In a manually operated solid-phase peptide synthesizer, the DMF-CH 2 Cl 2 (1:1) prepared Boc-Har (NO 2 )-OH (500 mg, 1.5 mmole) solution was shaken with the neutralized resin and DIC (235 μl, 1.5 mmole) for 1 hour. Aft...
Embodiment III
[0150] Embodiment III biological activity:
[0151] The peptides of the invention were tested in in vitro and in vivo assays to determine their inhibition of hGH-RH(1-29)NH 2 Ability to induce GH release. Hypocooled rat pituitary system:
[0152] These peptide analogs were tested according to a modification (Z. Rekasi and A.V. Schally, P.N.A.S. 90: 2146-2148, 1993) of an assay previously described (S. Vigh and A.V. Schally, Peptide 5: 241-347, 1984). In vitro assays were performed.
[0153] Briefly, cells were pre-incubated with various concentrations of peptides (3ml) for 9 minutes. Add 1nM hGH-RH(1-29)NH immediately after incubation 2 (1 ml) for 3 minutes [0 minute response]. To examine the duration of the antagonistic effect of this analogue, 1 nM hGH-RH(1-29)NH was added after 30, 60, 90 and 120 minutes 2 , for 3 minutes [30, 60, 90, 120 minute responses]. The net integer value of the GH response was estimated. Compare GH responses with 1 nM hGH-RH(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com