Methods and apparatus for extrusion of vesicles at high pressure
A vesicle, high-pressure technology, applied in pharmaceutical formulations, microcapsule preparations, medical preparations of inactive ingredients, etc., can solve the possibility of difficult lipid extrusion, low flow rate, increased production time and cost pollution, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
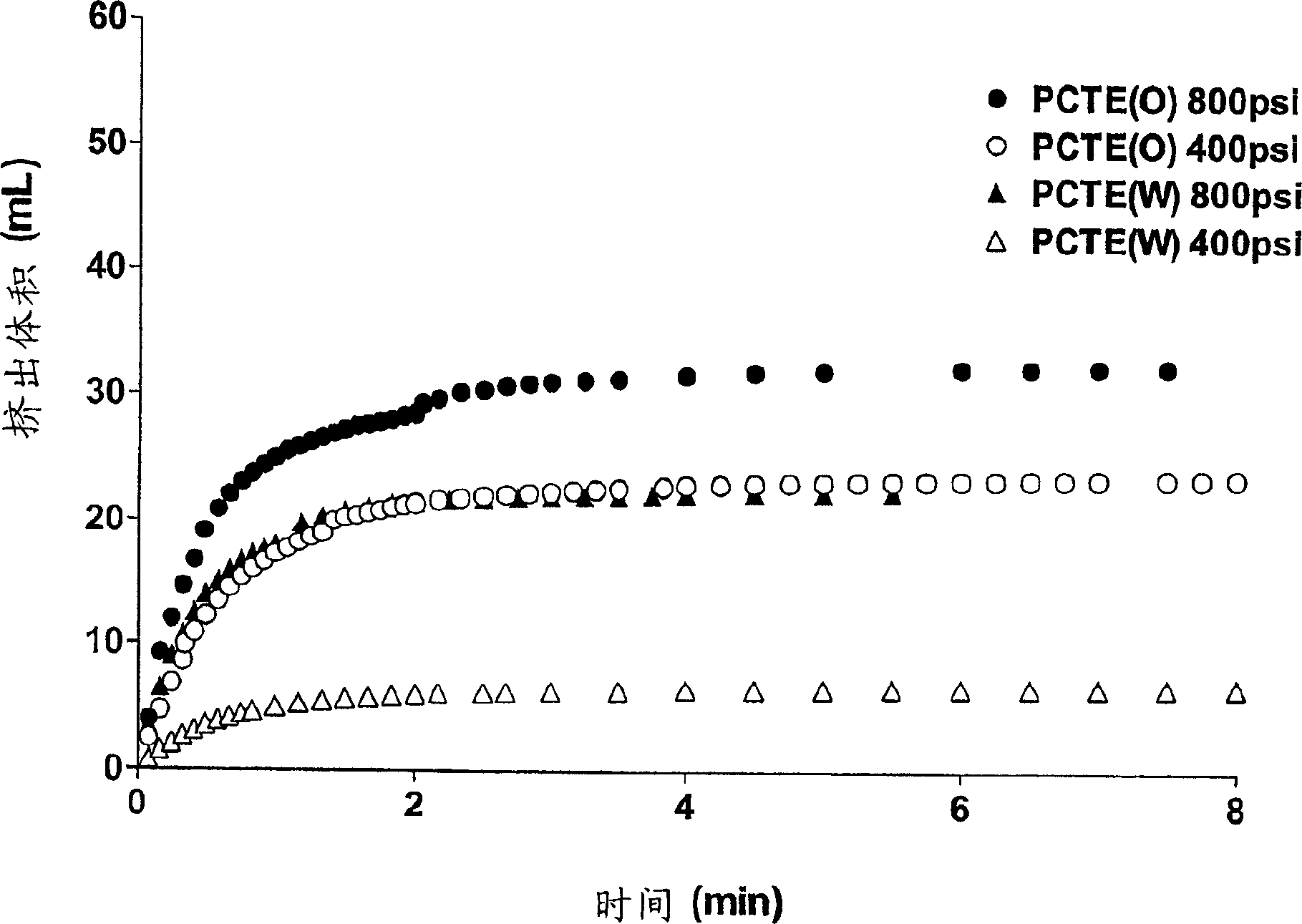
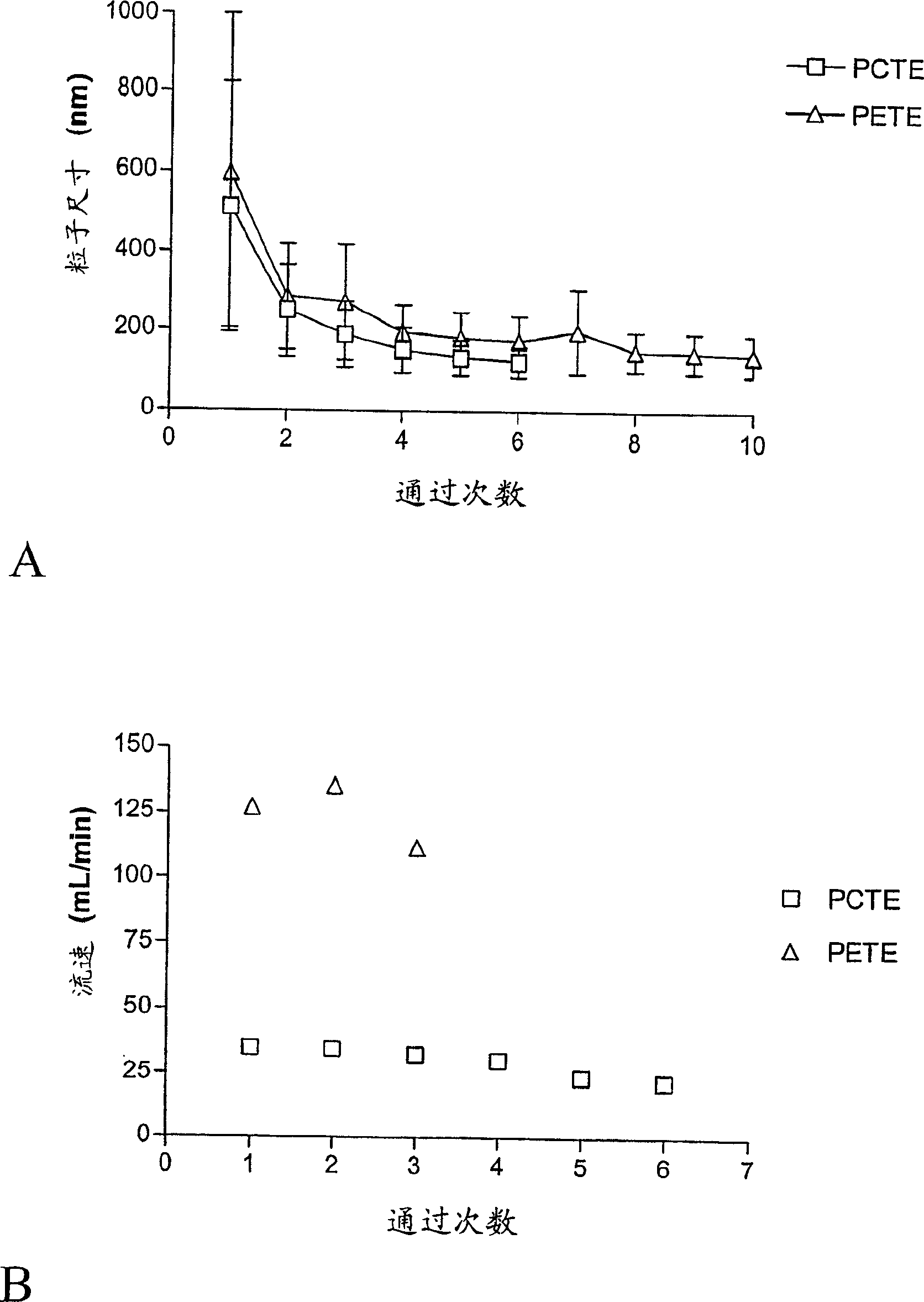
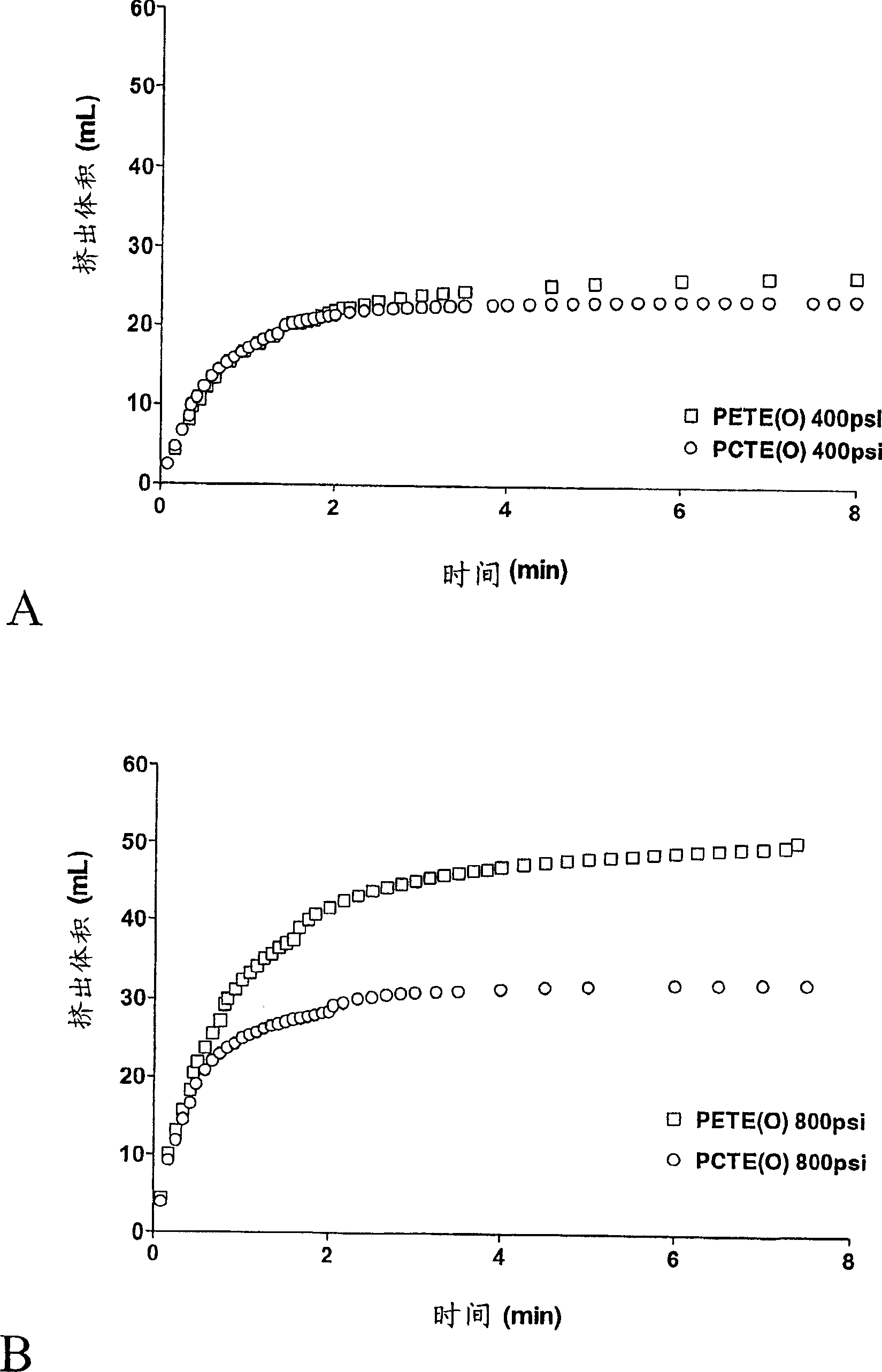
[0100] 1. Extrude 20% POPC through a 0.1 μm polycarbonate track-etched membrane at 600 psi
[0101] The following examples demonstrate that, using conventional methods and apparatus, difficult-to-extrude lipids can clog or foul extruded membranes if cleaning agents are not advantageously used.
[0102] In a 50 ml conical tube, add 2 g of 1-palmitoyl-2-oleoyl-sn-propanetrioxy-3-phosphocholine (POPC) (Genzyme, Cambridge MA, cat. no. LP-04-031) Add 8 ml of phosphate-buffered saline solution (PBS) (140 mM saline, 20 mM phosphate, pH=7.4), and shake vigorously by hand for about 5 minutes to form a uniform suspension of 200 mg / ml POPC MLVs in PBS. Follow the extruder manufacturer's instructions in 10 ml of LIPEX TM 2-stacked 0.1 μm polycarbonate track-etched membrane (PCTE) NUCLEPORE mounted on an extruder (Northern Lipids, Vancouver, British Columbia, Canada) TM membrane (Whatman, Ann Arbor, MI; catalog: 110605) and rinsed with PBS. The POPC-PBS MLV suspension was passed through...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| water contact angle | aaaaa | aaaaa |
| water contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com