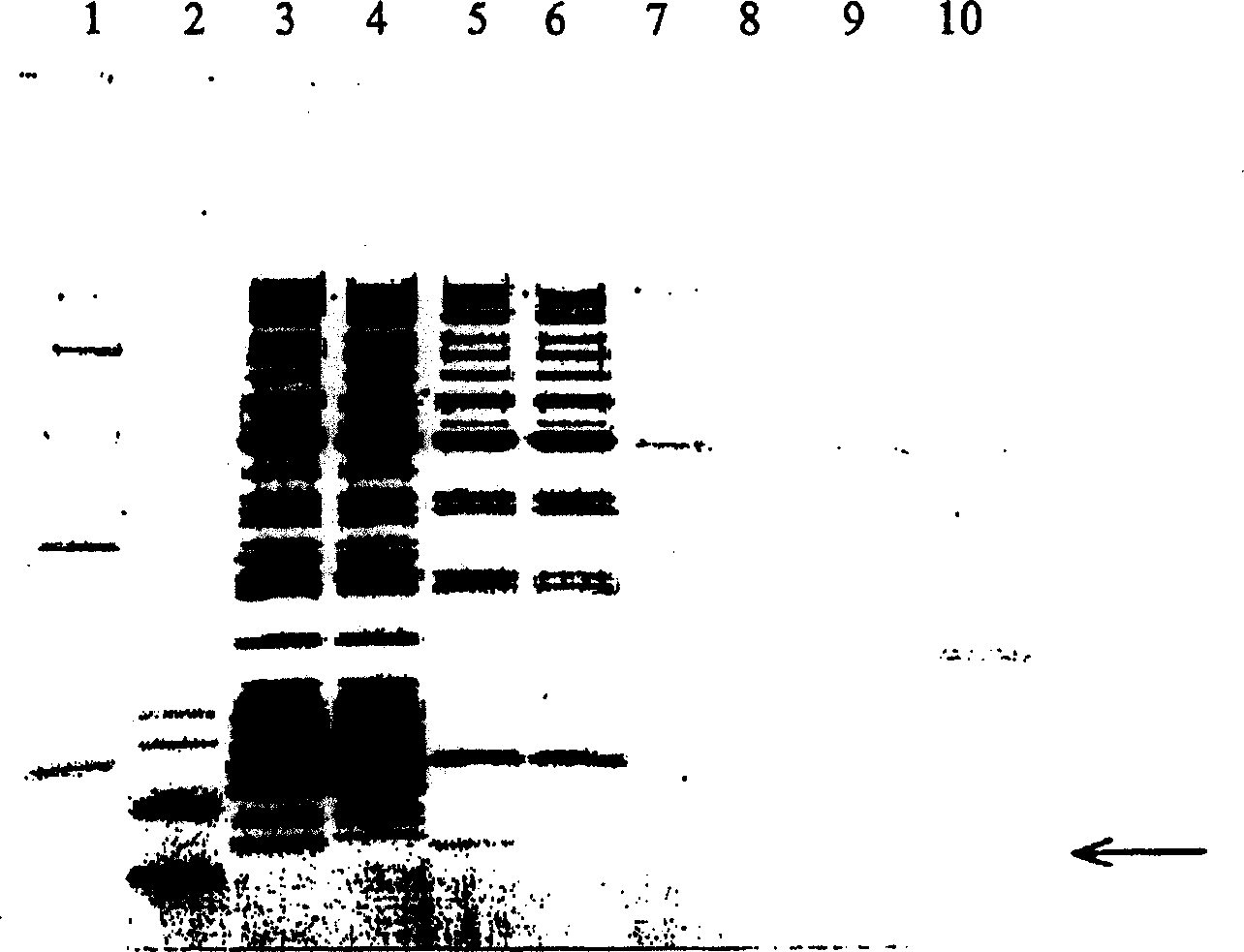Antimicrobial polypeptide and utizliation thereof
一种抗菌性、肽链的技术,应用在抗菌剂,抗菌性多肽领域,能够解决难以开发出抗菌活性抗菌性能抗菌剂等问题,达到高抗菌活性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
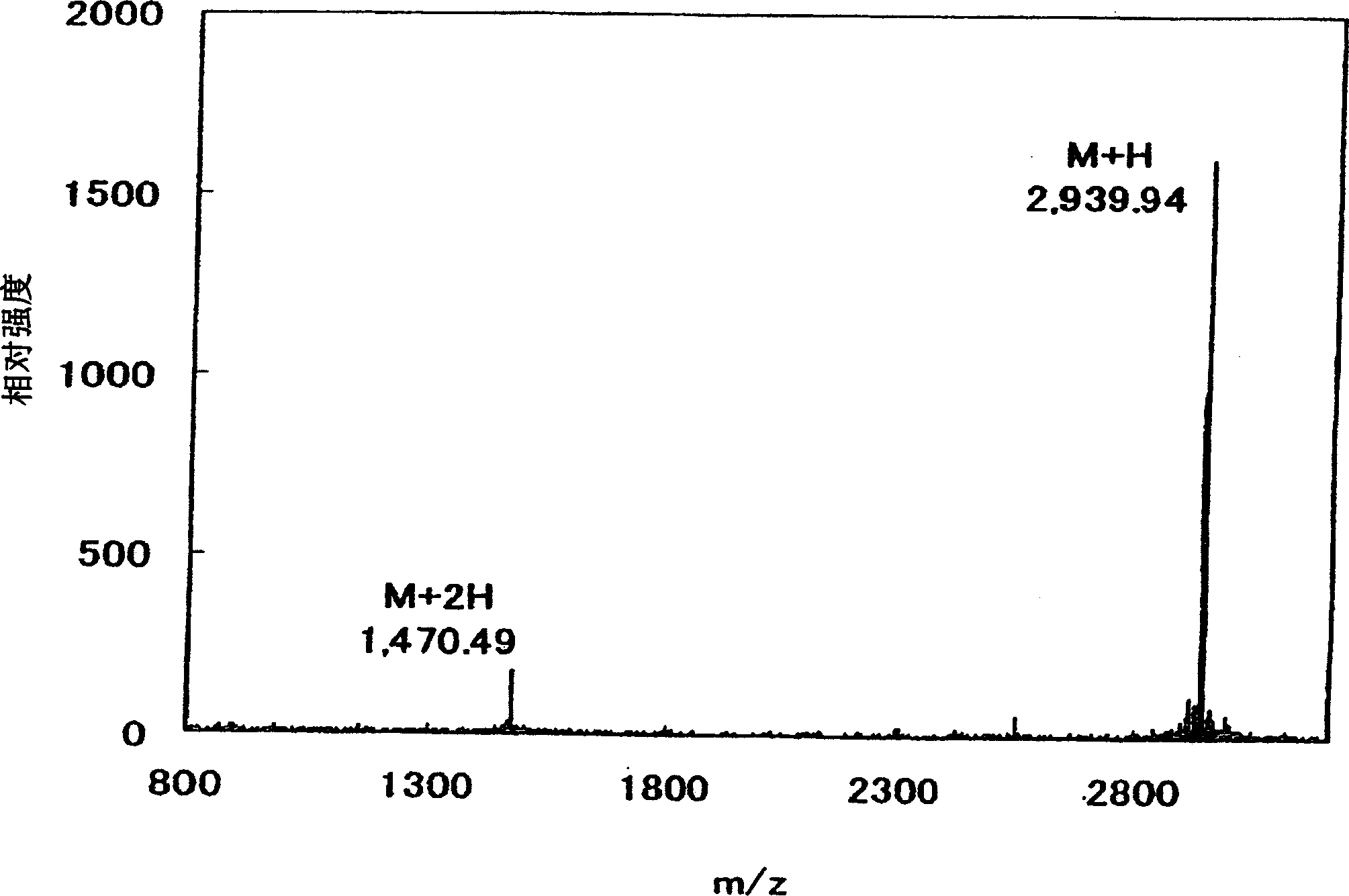
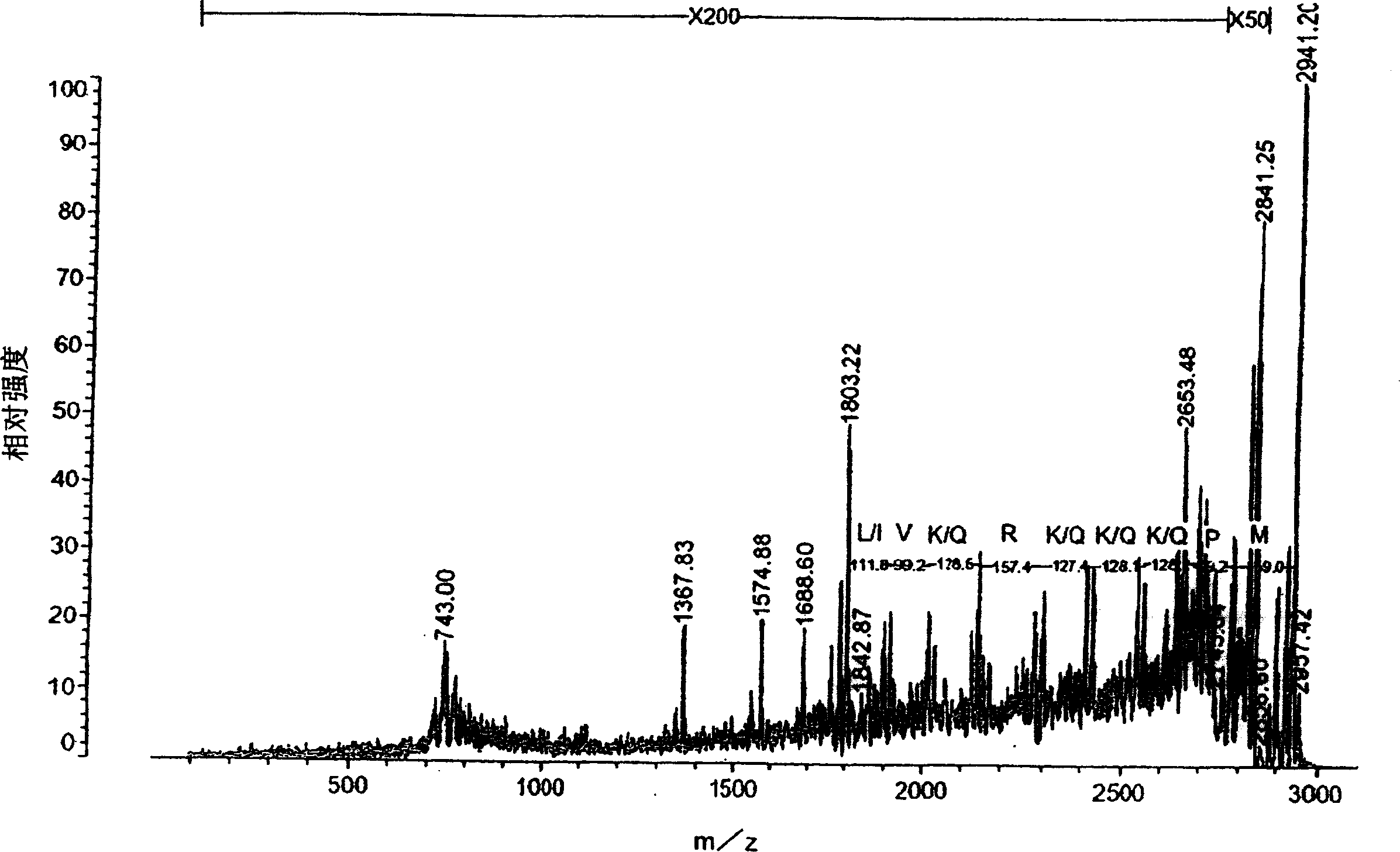
Image
Examples
Embodiment 1
[0123] Embodiment 1: the chemical synthesis of antibacterial polypeptide
[0124] A total of 36 kinds of polypeptides (samples 1 to 33, reference products 1 to 3) were produced using the peptide synthesizer described below. Table 1 lists the amino acid sequences of these polypeptides.
[0125] Sample serial number
amino acid sequence
total number of amino acid residues
sample 1
PKKKRKV (serial number 4)
7
sample 2
RQARRNRRRRWR (serial number 21)
12
sample 3
RIRKKLR (serial number 43)
7
Sample 4
PPRKKRTVV (serial number 28)
9
Sample 5
RKKRRQRRR (serial number 20)
9
Sample 6
PRRRK (serial number 26)
5
Sample 7
RKKKRKV (serial number 83)
7
Sample 8
PKKKRKVLPPLERLTL (serial number 84)
16
Sample 9
LPPLERLTLPKKKRKV (serial number 85)
16
sample 10
RQARRNRRRRWRLPPPLERLTLD (serial number ...
Embodiment 2
[0151] Embodiment 2: the antibacterial activity of synthetic polypeptide
[0152] With 96 wells (well) microculture plate, obtain antibacterial property polypeptide (sample 1~33) of the present invention and the polypeptide of reference product 1~3 by liquid medium dilution method to Gram-negative bacteria (Escherichia coli: Escherichia coli: E. .coli) and Gram-positive bacteria (Staphylococcus aureus: S.aureus) antibacterial activity (minimum inhibitory concentration: MIC).
[0153] That is, liquid gravy medium (DIFCO product "NUTRIENTBROTH Dehydrated") with peptide concentrations of 500, 250, 125, 62.5, 31.3, 15.6, 7.8, 3.9, 1.9, 1.0, and 0.5 μM were prepared and filled into 96-well microculture plate. In addition, the bacterial liquid (approximately 2 × 10 6 cells / mL) and the drug solution (the above-mentioned peptide-containing broth medium) were inoculated in each well of a 96-well microplate. After inoculation, culture was started in a 37°C incubator, and sterility wa...
Embodiment 3
[0160] Embodiment 3: antibacterial polypeptide
[0161] Then, using various bacteria (see Table 3) as objects, the same antibacterial test (MIC measurement test) as in Example 2 was performed on the polypeptides of samples 16, 17, 10, 14 and 20, and the antibacterial spectrum of these polypeptides was evaluated. The results are shown in Table 3, and it was confirmed that the antibacterial polypeptide of the present invention has broad-spectrum antibacterial activity against both Gram-negative bacteria and Gram-positive bacteria.
[0162] Test bacteria
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com