Cancer vaccines and methods of using the same
A technology for methylating oligonucleotides and methylating nucleic acids, which can be applied to biochemical equipment and methods, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the problem of unobserved dendrites. Cellular immune stimulatory activity, not provided, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0156] Preparation of the composition
[0157] Preparation of the compositions of the present invention may be accomplished by any technique, but most preferably alcohol dialysis or detergent dialysis are described in detail in the following publications, patents and applications, all of which are hereby incorporated by reference: U.S. Patent 5,705,385; Patent 5,976,567; US Patent Application 09 / 140,476; US Patent 5,981,501; US Patent 6,287,591; International Publication No. WO96 / 40964 and International Publication No. WO98 / 51278. These manufacturing methods provide for small-scale and large-scale manufacture of therapeutic agent-containing immunostimulatory compositions encapsulated in lipid particles, preferably lipid-nucleic acid particles. These methods also yield particles with excellent pharmaceutical properties.
[0158] Vaccine compositions of the present invention can be prepared by adding the target antigen where a response is desired. Methods for incorporation o...
Embodiment 1
[0205] Comparison of in vitro and in vivo leukocyte activation by exposure to free or encapsulated oligonucleotides in whole blood cells
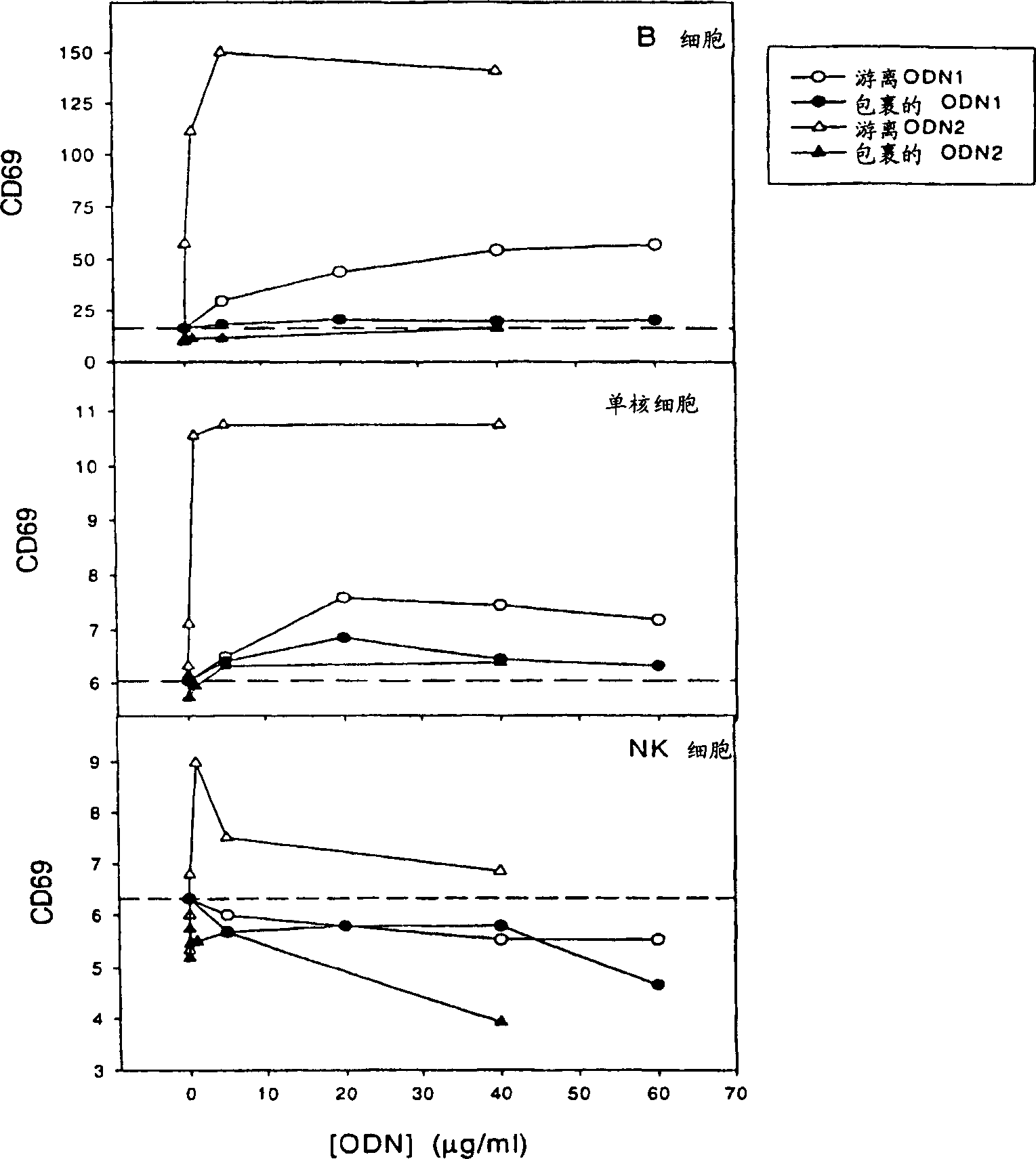
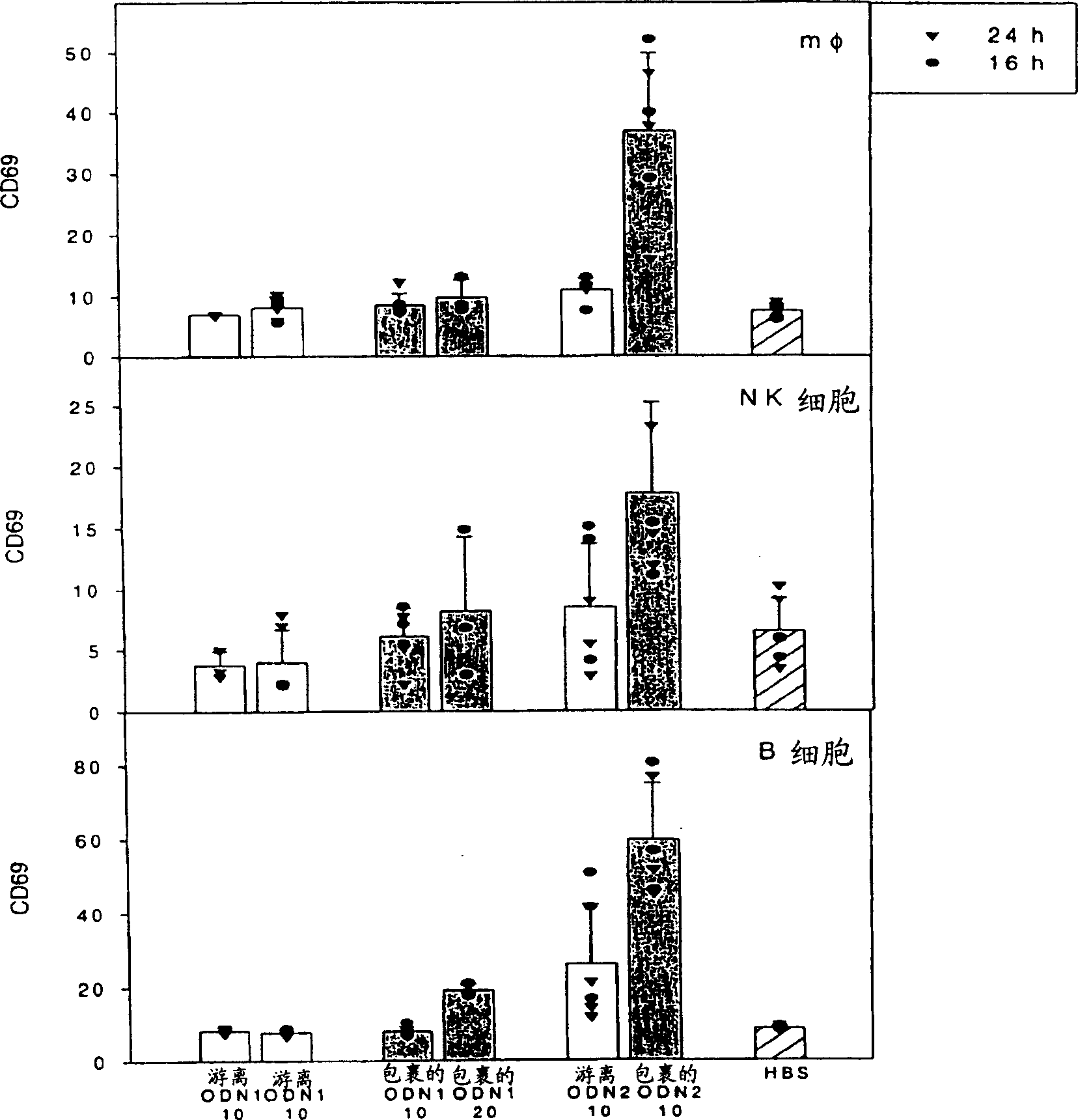
[0206] To indicate the potency of in vitro assays for predicted immune stimulation in vivo, CD69 expression was compared in figure 1 and figure 2 displayed in . CD69 is a cell activation marker, quantitatively expressing the activity of NK cells, B cells and monocytes. The expression of CD69 in NK cells indicates cell activation and IFN-g production, which is important for the induction of Th-1 immune responses. The ability of free and encapsulated ODN1 and 2 to induce CD69 expression was tested in vitro and in vivo. In vitro, the dose was 0.1 mg / ml ODN2 and 10 mg / ml ODN1, and in vivo, the dose was 10 mg / kg ODN2 and 20 mg / kg ODN1. Each oligonucleotide was encapsulated in lipid particles consisting of POPC:CHOL:DODMA:PEGDMG in a ratio of 20:45:25:10.
[0207] figure 1 showed in vitro stimulation of leukocytes containing the activation...
Embodiment 2
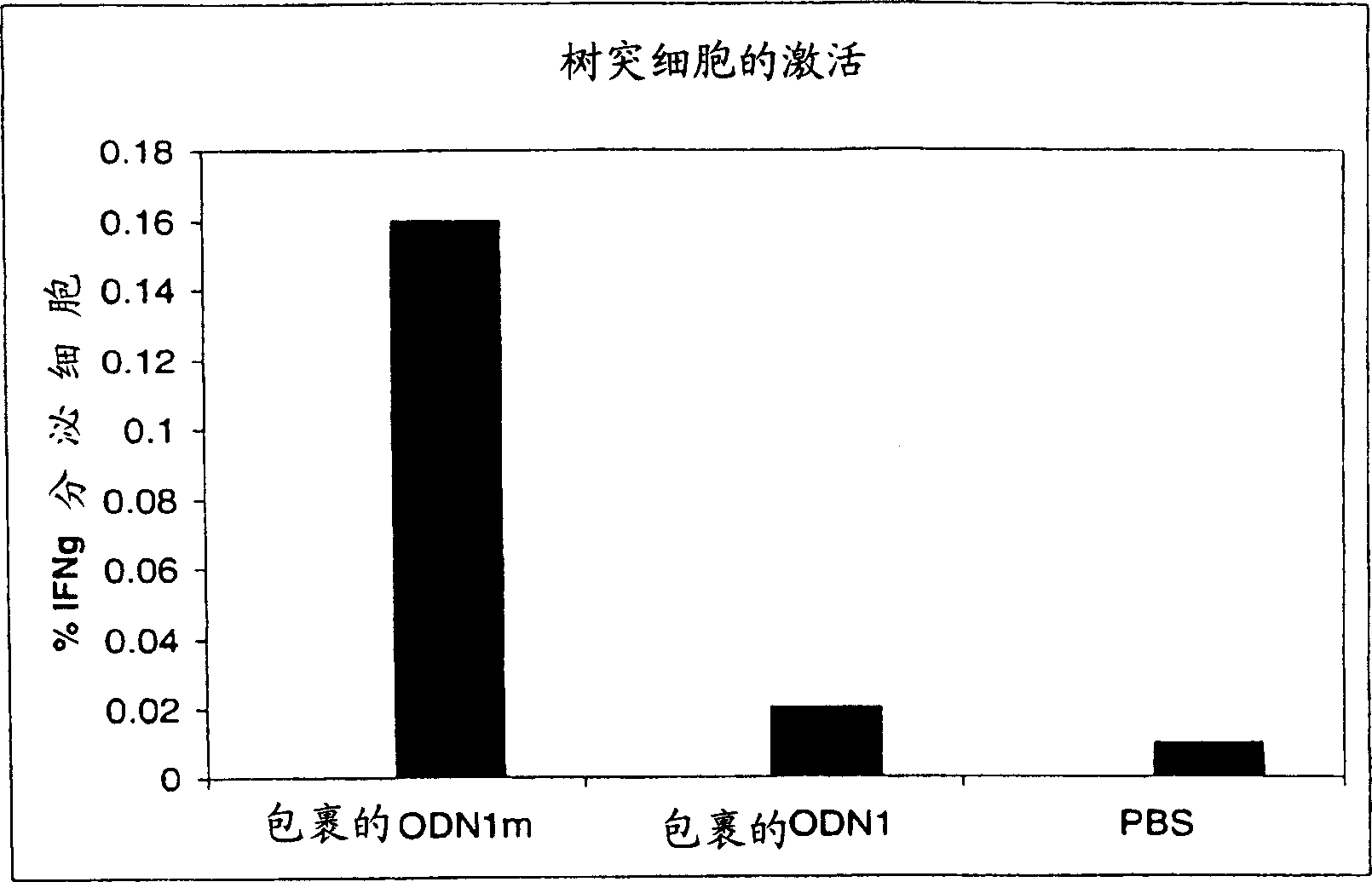
[0211] In vivo, dendritic cell activation with methylated oligonucleotides
[0212] As noted above in the Background section, the prior art teaches that methylated CpG oligonucleotides are generally less effective at stimulating an immune response than unmethylated CpG oligonucleotides, whether measured in vivo or in vitro, or Less potent than unmethylated CpG oligonucleotides. US Patent 6,429,199 discloses that methylated oligonucleotides do not enhance CD40 expression in NK cells or human B cells, and do not exhibit any improved survival of dendritic cells, which are involved in Th-1 responses in body fluids and cells Major antigen-presenting cells in immunity. Furthermore, the disclosed methylated CpG oligonucleotides did not enhance the survival, differentiation, activation or maturation of dendritic cells in vitro. Similarly, the in vitro PBMC results published in WO02 / 069369 also did not show any activity of methylated oligonucleotides on dendritic cells.
[0213] In ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com