Process for coating a pharmaceutical particle
A technology of coating and granulation, which is applied in the direction of coating granules, granulation of raw materials, drug combination, etc., which can solve the problems of poor protection, pungent smell, and low recovery efficiency of intestinal products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
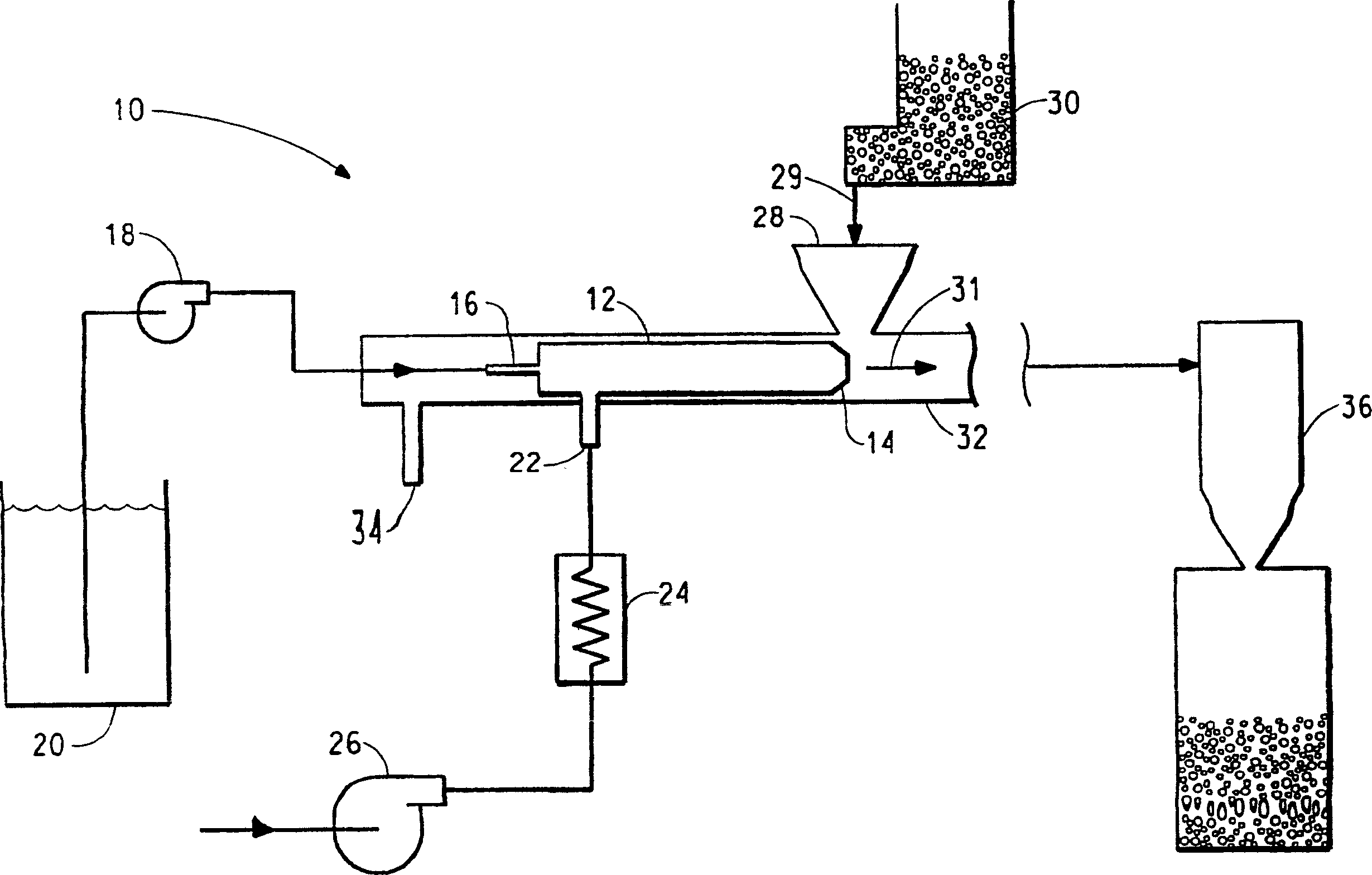
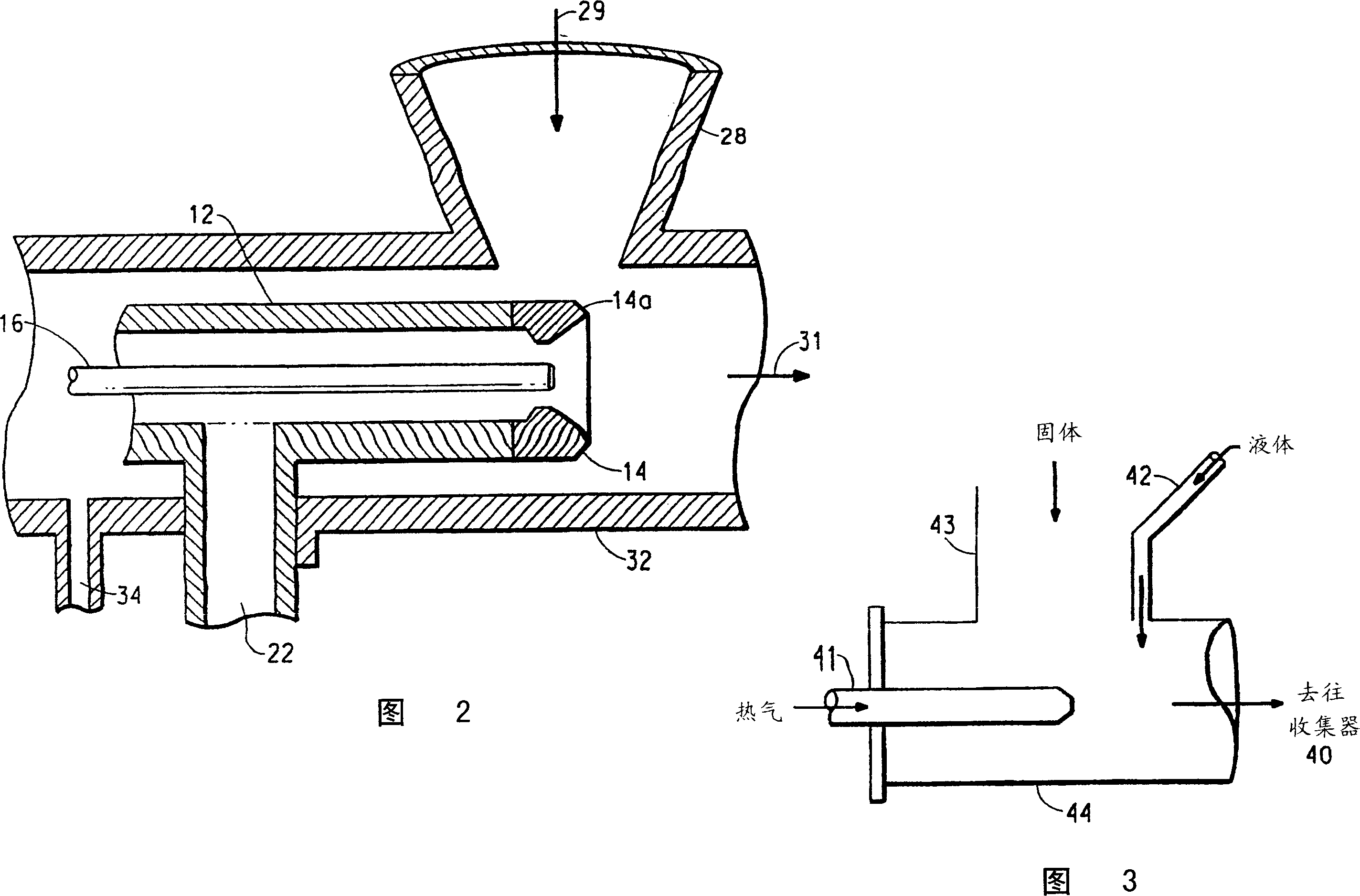
[0112] use as figure 1 The device shown was coated with a sample of ibuprofen USP (Spectrum Chemical Co., Gardena, CA). This device had a 3.18 cm diameter mixing chamber 19.05 cm long with a 1.02 cm nozzle throat and a 0.39 cm diameter central liquid feed tube. This unit has a single screw metering feeder (AccuRate, Whitewater, WI) for metering solid particles. A peristaltic pump (Cole-Parmer, Vernon Hill IL) was fitted with a 6.5mm Tygon TM Elastic rubber tube for metering liquids. Ibuprofen was metered into the system (51.3, 71.6, 120.5 g / min). Eudragit RL30D was metered into the base tube at a range (27.0, 28.1, 30.4 g / min) at an ambient temperature of 22°C. The pressure of the heating gas at the nozzle was 551 kPa, and the temperature at the nozzle was 125°C. Pressurized nitrogen is used to nebulize Eudragit RL30D, create a negative pressure in the mixing area to promote the addition of ibuprofen, and provide heat to evaporate the residual water on the ibuprofen...
Embodiment 2
[0118] use as figure 1 The device shown is coated with ibuprofen USP (Spectrum Chemical Co., Gardena, CA). This device has a mixing chamber with a diameter of 2.54 cm and a length of 19.05 cm or a mixing chamber with a diameter of 3.18 cm and a length of 43.18 cm. The mixing chamber has a nozzle throat with a diameter of 0.64-1.02 cm and a diameter of 0.18-0.39 cm. Center liquid feed tube. This unit has a single-screw metering feeder (AccuRate) for metering solid particles. In this example, the ibuprofen feed rate was 300-400 g / min. Peristaltic pump (Masterflex model5718-10 Cole-Parmer, Vernon Hill. IL) equipped with Masterflex LS / 25 (4.8mmI.D) or Masterflex LS / 16 (3.1mmI.D) Tygon Elastic rubber tube for metering liquids. Ethylcellulose (Ethocel Standard, Premium; Dow Chemical Co., Midland, MI) was dissolved in acetone to form a coating solution. In some cases, triethyl citrate (as a plasticizer; Spectrum Chemical Co., Gardena, CA) was also dissolved in the solution. T...
Embodiment 3
[0124] use as figure 1The device shown and described in Example 2 was coated with caffeine, USP (Spectrum Chemical Co., Gardena, CA). This unit has a single screw metering feeder (AccuRate, Whitewater, WI) for metering solid particles. In this example, the ibuprofen feed rate was 300-400 g / min. Peristaltic pumps (Masterflex model 5718-10) were fitted with Masterflex LS / 25 (4.8mm I.D) or Masterflex LS / 16 (3.1mm I.D) Tygon elastic rubber tubing for metering liquids. Eudragit was dissolved in acetone to form a coating solution. In some cases, triethyl citrate (as a plasticizer) was also dissolved in the solution. The coating solution was metered into the base tube at room temperature in the range of 20-30 g / min. Heated nitrogen is used to atomize the coating solution, create negative pressure in the mixing zone to introduce caffeine, and provide heat to evaporate the solvent. The mixed / dried product goes through the mixing chamber into the cyclone separator to collect the pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com