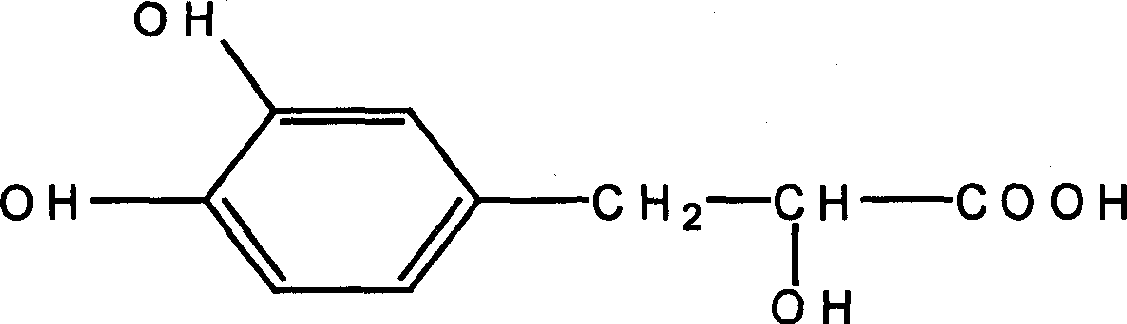
Application of Danshensu in preparation of medicine for treating cerebrovascular diseases
A technology of cerebrovascular disease and danshensu, applied in the field of application of danshensu in the preparation of drugs for treating cerebrovascular diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0016] Preparation Example 1: Preparation of Danshensu Freeze-dried Powder for Injection
[0017] Precisely weigh 20.0g of Danshensu raw material and 40.0g of medicinal mannitol, dissolve in 1000ml of water for injection, stir well to dissolve, add water for injection to 2000ml, add 2.0g of activated carbon for needles, heat to 60°C and stir for 30 minutes , carbon rod filtration, and the filtrate was sterilized by filtration through a 0.25 μm microporous membrane, divided into 1000 vials, with a filling volume of 2.0ml / vial, and freeze-dried to obtain the product. Each bottle contains danshensu 20mg.
preparation example 2
[0018] Preparation Example 2: Preparation of Danshensu Injection
[0019] Accurately weigh 20.0g of Danshensu raw material, dissolve in 1000ml of water for injection, stir well to dissolve, add water for injection to 2000ml, add 2.0g of activated carbon for needles, heat to 60°C and stir for 30 minutes, filter with carbon rod, and filtrate through Sterilize by filtration with a 0.25 μm microporous membrane, pack in 1,000 vials with a filling volume of 2.0 ml / bottle, seal, and sterilize. Each bottle contains danshensu 20mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com