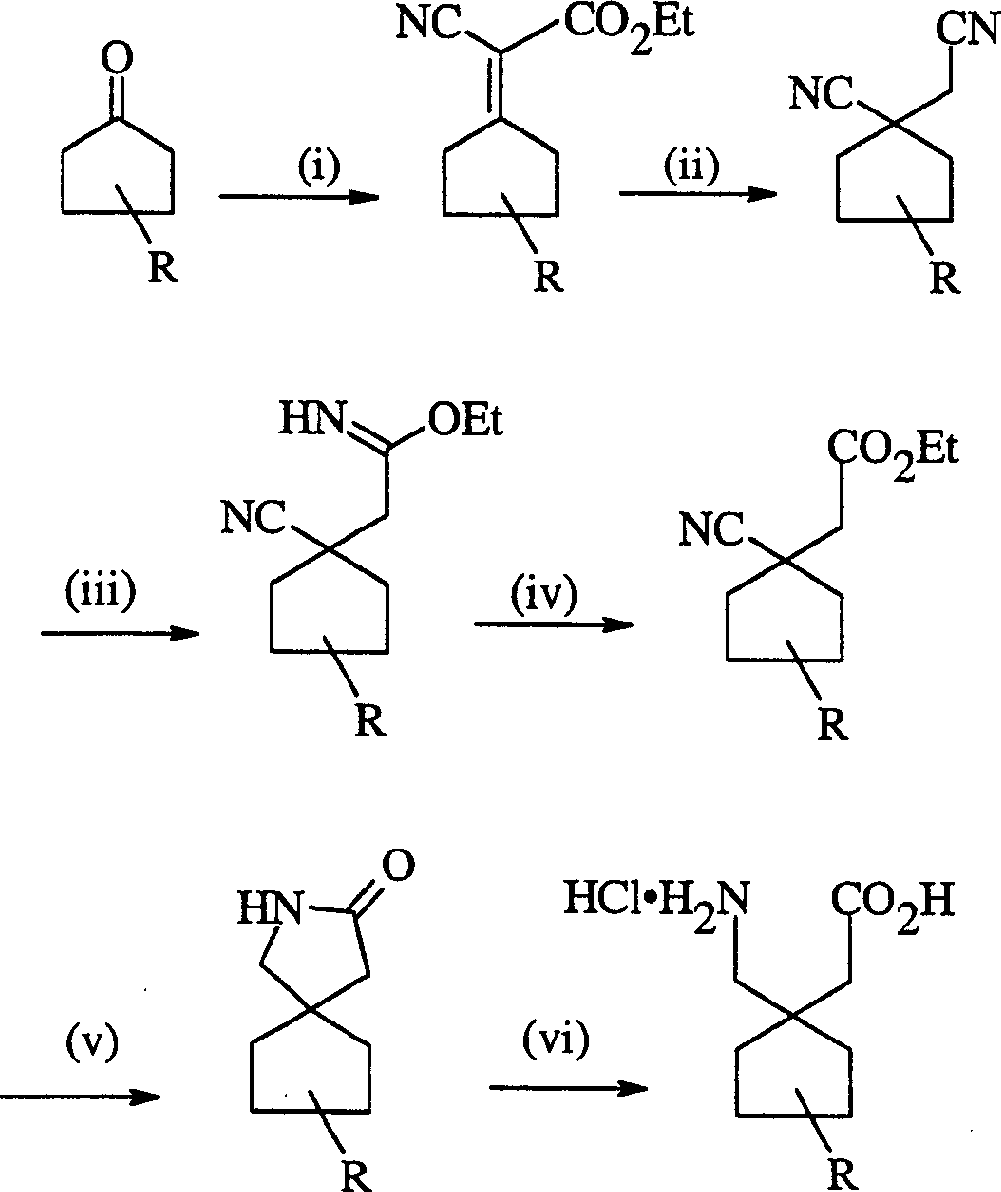
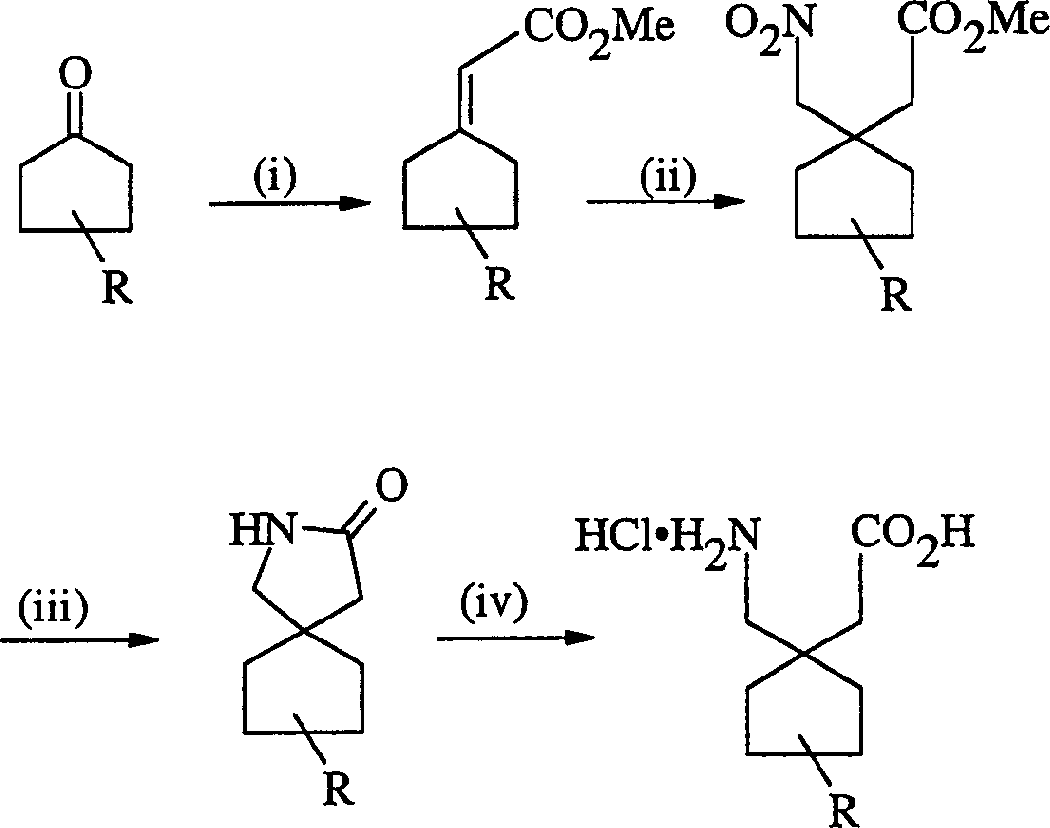
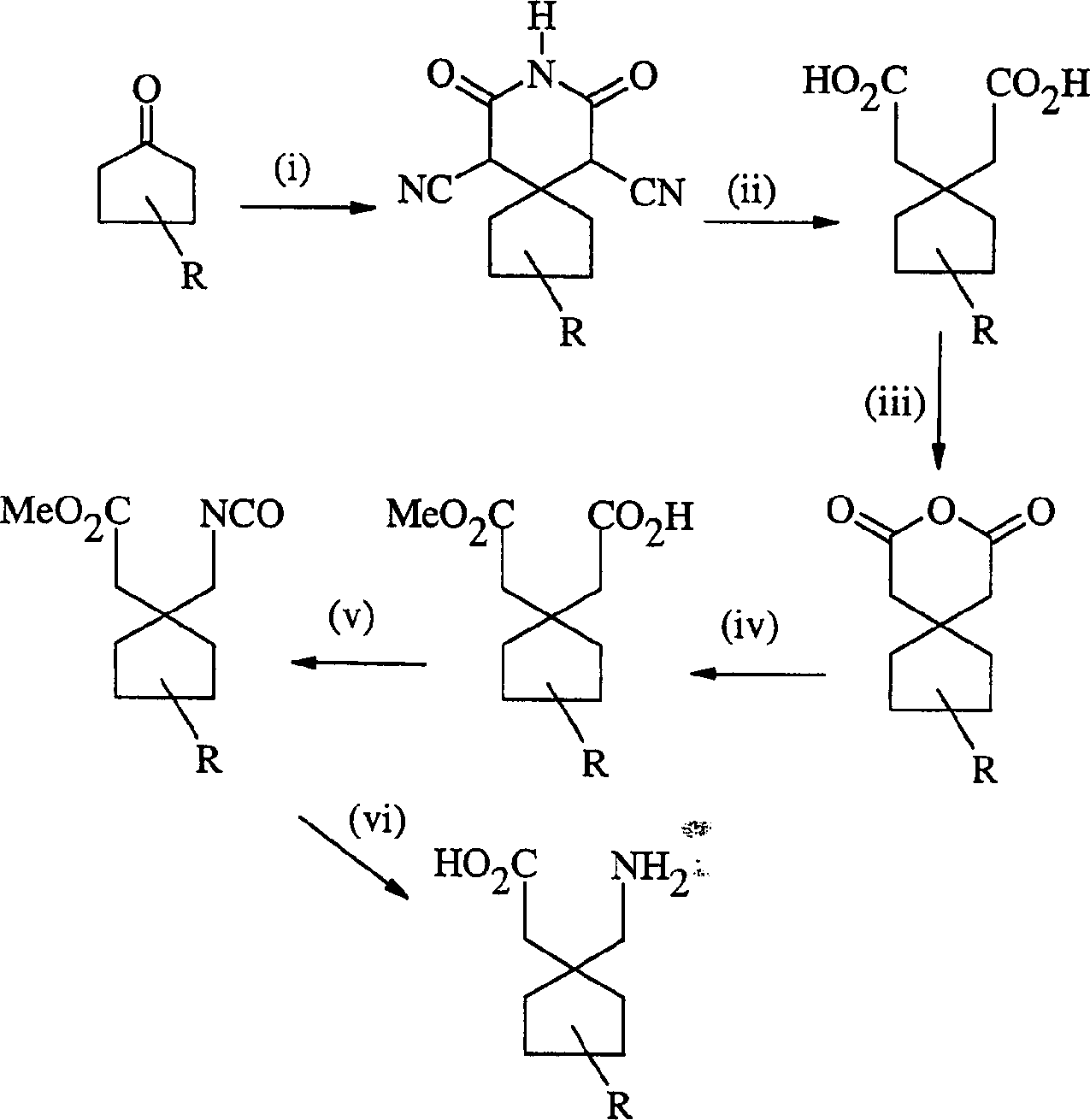
Gabapentin analogues for fibromy algia and other disorders
A human and disease technology, applied in the fields of muscular system diseases, neuromuscular system diseases, sexual diseases, etc., can solve the problems of disappointing clinical trial results, limited success of fibromyalgia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0399]
[0400] Reagents: (i) triethyl phosphonoacetate, NaH; (ii) MeNO 2 , Bu 4 N + f - ;(iii)H 2 , Ni; (iv) HCl
[0401] Synthesis of (trans)-(3,4-dimethylcyclopentylidene)-ethyl acetate (2)
[0402] NaH (60% dispersion in oil, 737mg, 18.42mmol) was suspended in anhydrous THF (50ml) and cooled to 0°C. Triethyl phosphonoacetate (3.83ml, 19.30mmol) was added, and the mixture was stirred at 0°C for 15 minutes. Ketone (1) (1.965 g, 17.54 mmol) in THF (10 ml) was then added and the mixture was allowed to warm to room temperature. After 2 hours, the mixture was partitioned between ether (200ml) and water (150ml). The organic phase was separated, washed with brine, dried (MgSO 4 ), and the solvent was removed in vacuo. The residue was purified by flash layer (silica gel, 1:9 ethyl acetate:heptane) to afford (2) 3.01 g (94%) as a colorless oil.
[0403] 1 H NMR 400MHz (CDCl 3 ): δ1.01 (3H, d, J = 6Hz), 1.03 (3H, d, J = 6Hz), 1.26 (3H, t, J = 7Hz), 1.49 (2H, m), 2.07 (...
Embodiment 2
[0420]
[0421] Reagents: (i) triethyl phosphonoacetate, NaH; (ii) MeNO 2 , Bu 4 N + f - ;(iii)H 2 , Ni; (iv) HCl
[0422] Synthesis of Ethyl Cyclobutylidene Acetate (2)
[0423] NaH (60% dispersion in oil, 1.80 g, 44.94 mmol) was suspended in anhydrous THF (80 ml) and cooled to 0°C. Triethyl phosphonoacetate (9.33ml, 47.08mmol) was added, and the mixture was stirred at 0°C for 15 minutes. Cyclobutanone (1) (3.0 g, 42.8 mmol) in THF (20 ml) was then added and the mixture was allowed to warm to room temperature. After 2 hours, the mixture was partitioned between ether (200ml) and water (150ml). The organic phase was separated, washed with brine, dried (MgSO 4 ) and the solvent was removed in vacuum at 600 mm Hg. The residue was purified by flash layer (silica gel, 1:19 ethyl acetate:pentane) to afford (2) 5.81 g (96%) as a colorless oil.
[0424] 1 H NMR 400MHz (CDCl 3 ): δ1.27(3H, t, J=6Hz), 2.09(2H, m), 2.82(2H, m), 3.15(2H, m), 4.14(2H, q, J=6Hz), 5.58(1H , s)....
Embodiment 3
[0438]
[0439] Reagents: (i) triethyl phosphonoacetate, NaH; (ii) MeNO 2 , Bu 4 N + f - ;(iii)H 2 , Ni; (iv) HCl
[0440] Synthesis of (R)-(3-methylcyclopentylidene)-ethyl acetate (2)
[0441] NaH (60% dispersion in oil, 1.86 g, 46.5 mmol) was suspended in anhydrous THF (40 ml) and cooled to 0°C. Triethyl phosphonoacetate (9.69ml, 48.8mmol) was added, and the mixture was stirred at 0°C for 15 minutes. Ketone (1) (5ml, 46.5mmol) in THF (10ml) was then added and the mixture was allowed to warm to room temperature. After 2 hours, the mixture was partitioned between ether (200ml) and water (150ml). The organic phase was separated, washed with brine, dried (MgSO 4 ), and the solvent was removed in vacuo. The residue was purified by flash layer (silica gel, 1:9 ethyl acetate:heptane) to afford (2) 5.45 g (70%) as a colorless oil.
[0442] 1 H NMR 400MHz (CDCl 3 ): δ1.04(3H, m), 1.27(3H, t, J=7Hz), 1.80-2.74(7H, m), 2.90-3.15(1H, m), 4.13(2H, q, J=7Hz) , 5.76 (1H, s)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com