Hepatitis B virus surface L protein related peptide
A hepatitis B and protein technology, applied in the direction of viral peptides, antiviral agents, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
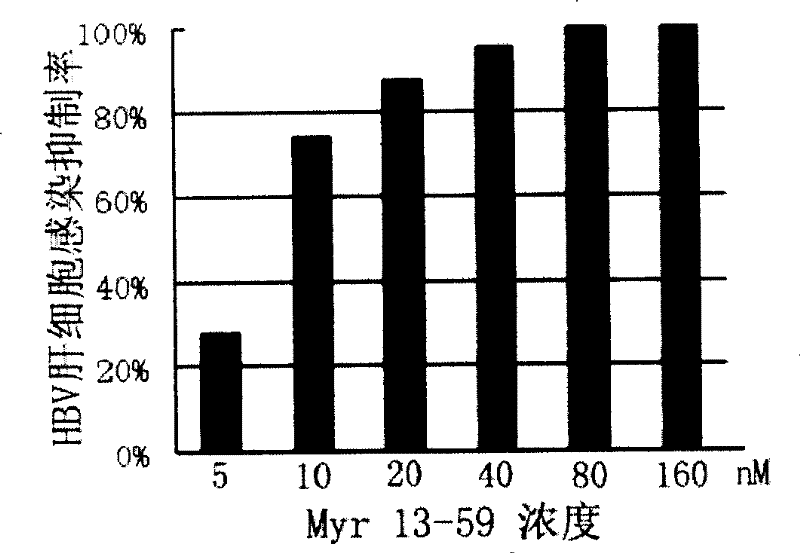
[0085] Example 1: Inhibition of HBV hepatocyte infection by peptides derived from HBV surface L protein of B genotype adw serotype and C genotype adr serotype
[0086] 1. Preparation of peptides derived from surface L protein of HBV serotype B genotype adw serotype and C genotype adr serotype
[0087] N-terminal myristoyl-modified HBV surface L protein-derived peptides of B genotype adw serotype and C genotype adr serotype (SEQ ID NO: 3 amino acid glycine modified with myristoyl, expressed as Myr13-59) as AB431A According to the standard Fmoc protocol, using 0.25mM HMP as the starting resin, the peptide synthesizer is extended and synthesized residue by residue from the carboxy-terminus to the amino-terminus. To enhance the stability of the polypeptide, the C-terminus of the polypeptide was further amidated. After peptide synthesis, cut with cutting solution, filter out resin with G6 glass sand funnel, and vacuum dry the filtrate. Ion-free water dissolves peptide cleavage pr...
Embodiment 2
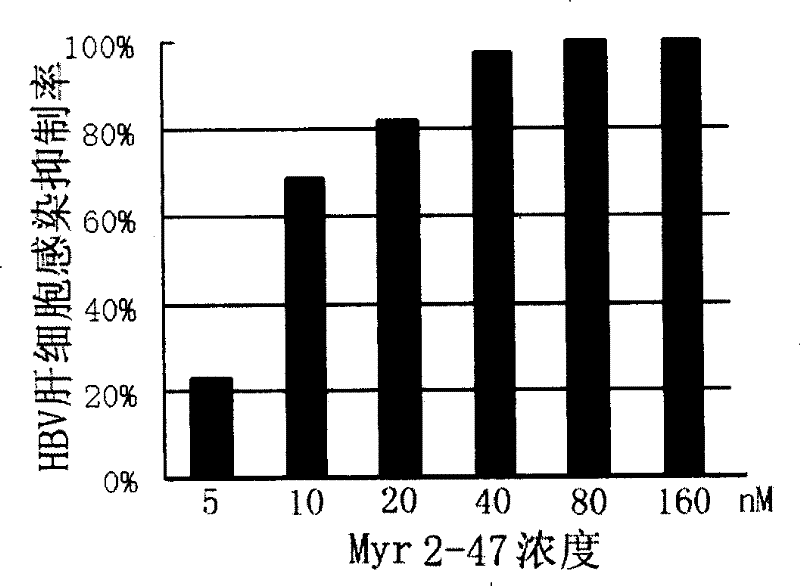
[0097] Example Two: Inhibition of HBV Hepatocyte Infection by Peptides Derived from Surface L Protein of D Genotype Ayw Serotype HBV Virus
[0098] 1. The synthesis of Myr2-47 (SEQ ID NO: 9) derived from surface L protein of HBV serotype D ayw serotype D with myristoyl modification (SEQ ID NO: 9) and the culture of primary human hepatocytes are the same as in Example 1.
[0099] 2. Cultivation of D genotype ayw serotype HBV virus
[0100] The 2.2.15 cell line, which can secrete complete D genotype ayw serotype HBV infected virus particles, was continuously cultured for 10 days. The culture supernatant was collected, centrifuged with 6% polyethylene glycol (PEG8000) to precipitate virus particles, the pellet was resuspended in phosphate buffer containing 25% fetal calf serum, and frozen at -80°C.
[0101] 3. Detection of HBsAg in hepatocyte culture supernatant after HBV infection (same as embodiment one)
[0102] 4. Inhibition of HBV surface L protein-derived peptide Myr2-47 ...
Embodiment 3
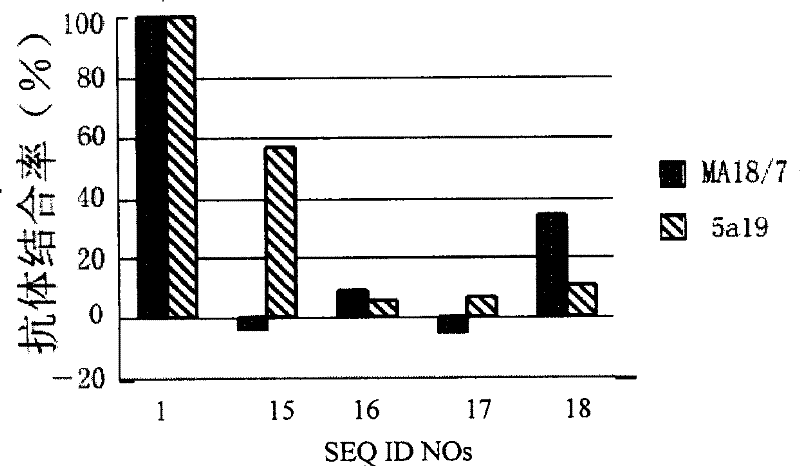
[0104] Example 3: Screening of HBV surface L protein derivatized peptides
[0105] 1, B genotype adw serotype and C genotype adr serotype HBV virus genotype serotype determination, virus culture, cultivation of human primary hepatocytes (same example 1).
[0106] 2. The construction of a recombinant plasmid carrying a complete HBV genome and having the complete replication ability of HBV.
[0107] Add 50 μl of HBV virus culture suspension to 310 μl proteinase K lysate [1mg / ml proteinase K, 50mmol / L Tris-HCl (pH8.0), 200mmol / L NaCl, 10mmol / L EDTA, 2% SDS, 1μg / ml poly(A) ]. After lysis at 60°C for 1 hour, extract with phenol / chloroform, precipitate with ethanol, and dissolve the precipitate in H 2 O is HBV DNA solution. Entire EN II replication element was amplified with upstream primer (Pst I) 5'ctgactgcagCACTGGATGGGGCTTGGCTATTGG (SEQ ID NO:21, 1202-1225) and downstream primer (EcoR I) 5'ttatggaattcCGACGCGGCGATTGAGACCTTC (2201-2180, SEQ ID NO:22). C genotype adr serotype HB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com