Sustained release device and method for ocular delivery of adrenergic agents
An adrenaline, sustained release technology, applied in the direction of capsule delivery, drug delivery, pill delivery, etc., can solve the problem that it is difficult to achieve zero release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
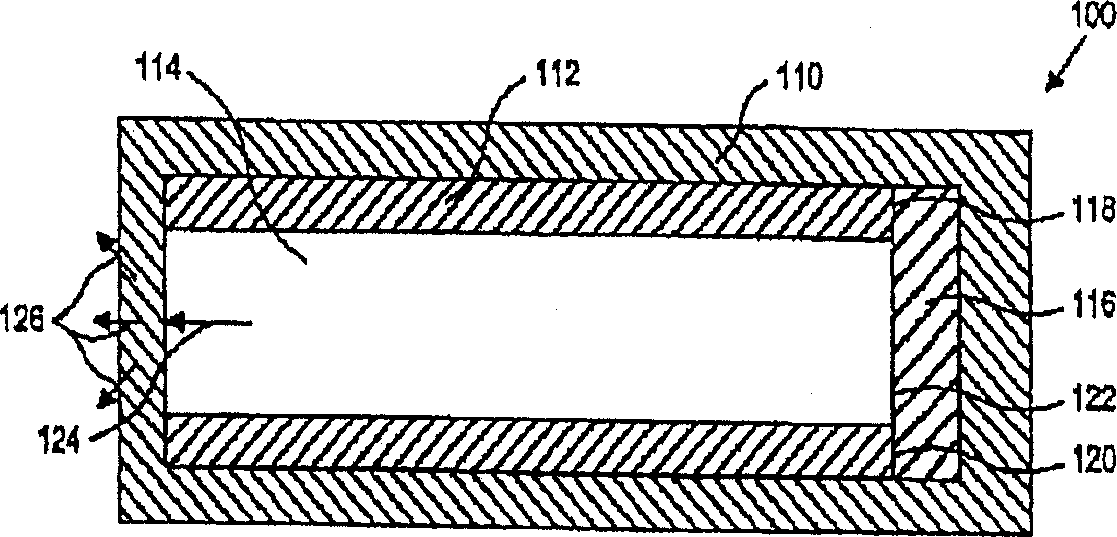
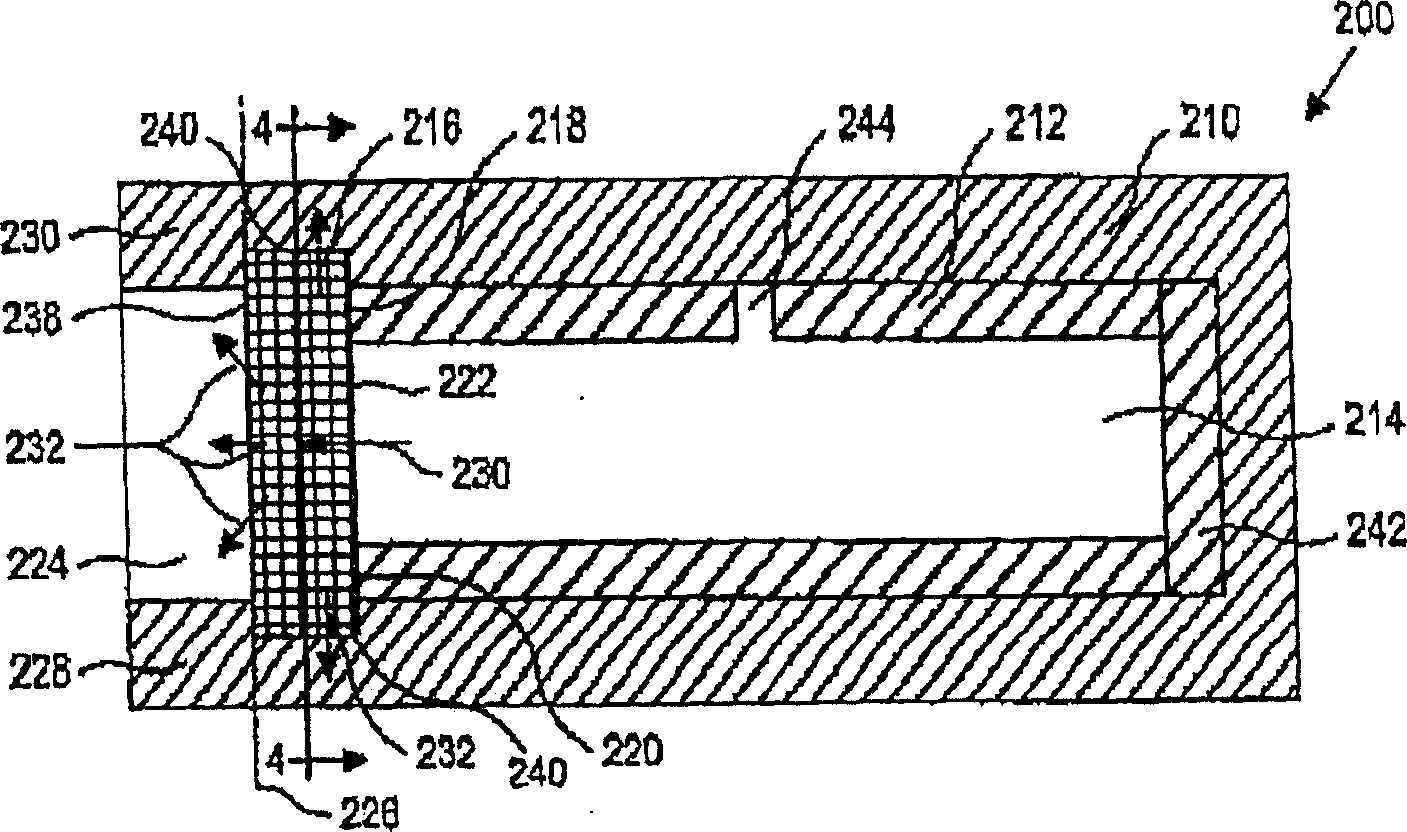
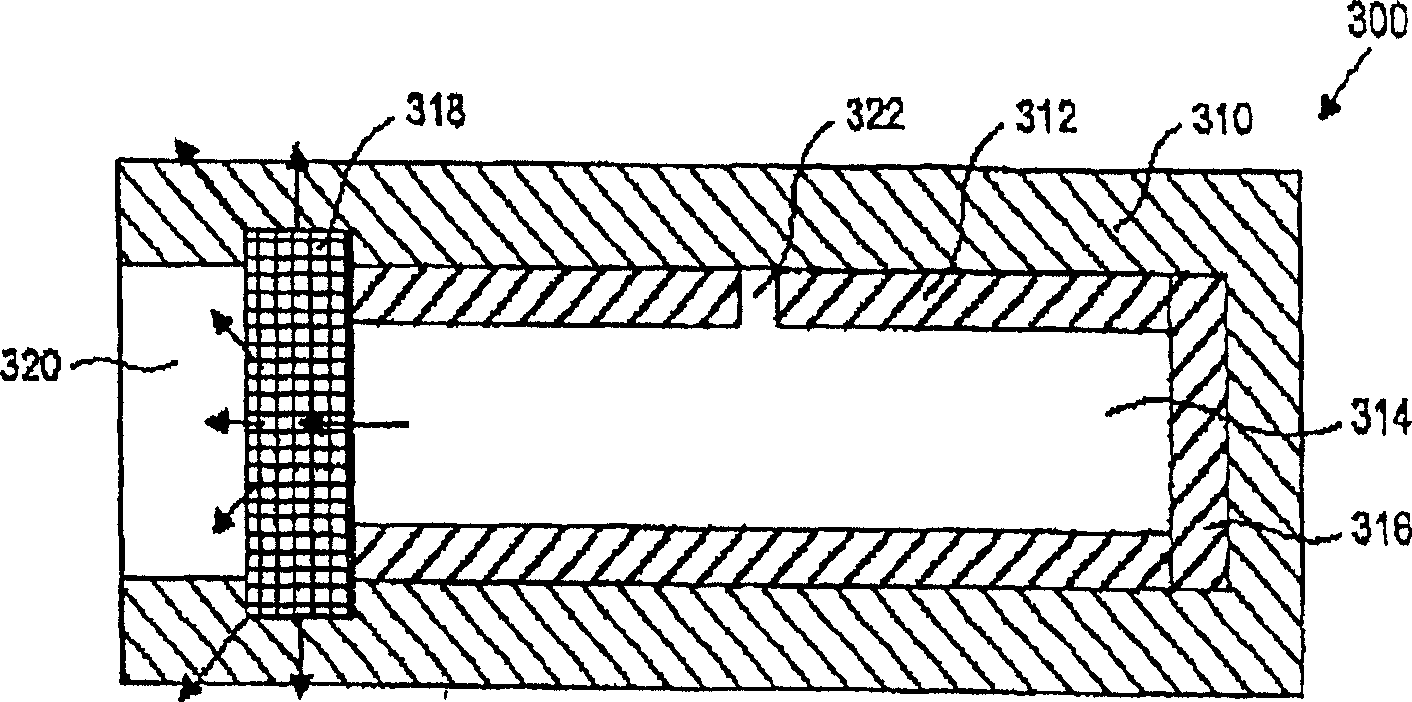
[0025] The present invention provides devices and methods for delivering and maintaining a therapeutic amount of at least one adrenergic agent to the ciliary body of a patient's eye for an extended period of time. The device is a sustained release drug delivery device comprising at least one adrenergic agent capable of maintaining a therapeutically effective concentration of the adrenergic agent in the ciliary body for an extended period of time. The method includes inserting the device into or near the eye of the patient to deliver the adrenergic agent to the ciliary body.
[0026]The device of the invention may be adapted for insertion into the eye and between the eyelids, preferably the lower eyelids. In other embodiments it may be adapted for insertion into the subretinal anterior or posterior chamber, into the choroid, or into or on the sclera. In another embodiment, the device may be adapted for insertion into a lacrimal canaliculus. In another embodiment, the device m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com