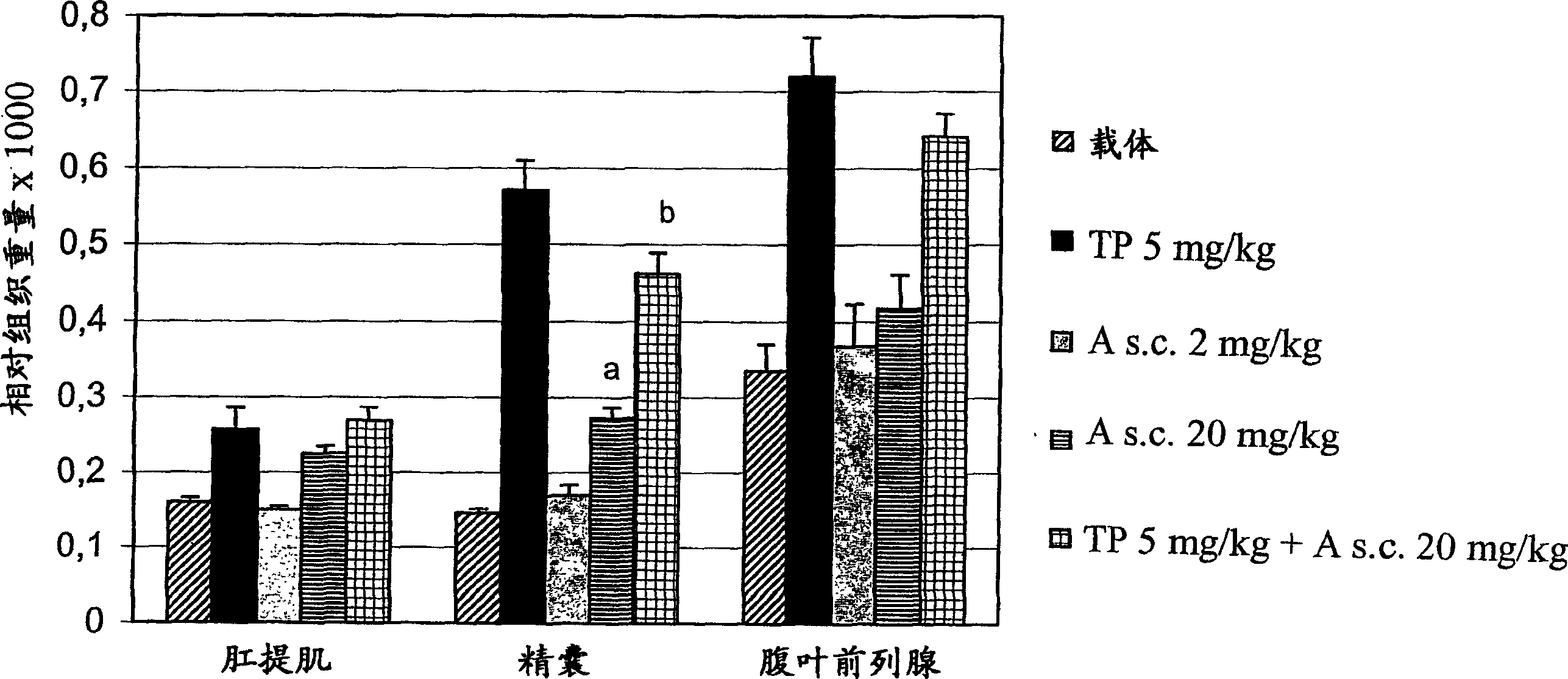
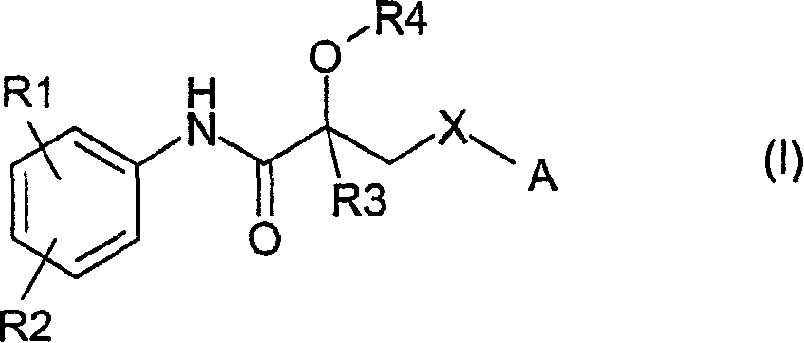
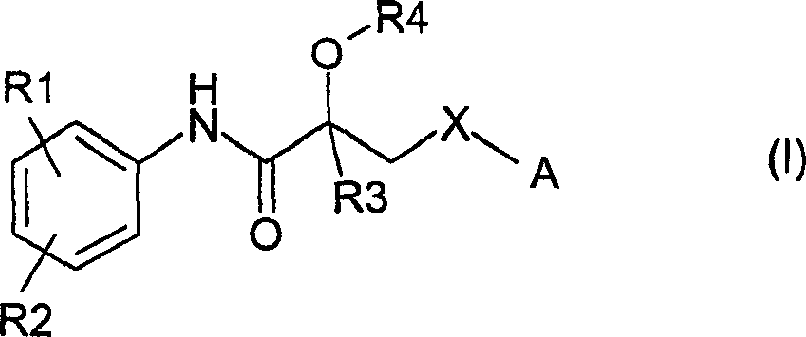
Propionamide derivatives useful as androgen receptor modulators
A halogen, compound technology, applied in the field of tissue-selective androgen receptor modulators, pharmaceutical compositions containing such compounds, therapeutic active compounds for androgen receptor-dependent disorders, and pharmaceutically acceptable salts and esters thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1. (Method A)
[0106] 3-(4-Acetamidophenoxy)-2-hydroxy-2-methyl-N-(3-methyl-4-nitrophenyl)-propionamide
[0107] a) 2-methyl-N-(3-methyl-4-nitrophenyl)acrylamide
[0108] 3-Methyl-4-nitroaniline (2.0 g, 13 mmol) in N,N-dimethylacetamide (DMAC) (6 ml) was added dropwise to cold methacryloyl chloride (2.0 ml, 20.7 mmol) while maintaining the temperature of the reaction mixture at 0-5 °C. The solution was allowed to warm to room temperature and the mixture was stirred overnight. The mixture was poured into water (70ml) and extracted with ethyl acetate (4x40ml). The organic phase was saturated with Na 2 CO 3 (3×20ml) and water (1×50ml), washed with Na 2 SO 4 Dry and evaporate. The crude yield was 4.17 g (with DMA, theoretical yield 2.9 g), which was used without further purification.
[0109] 1 H NMR (DMSO-d 6 ): 1.97(3H,s), 2.55(3H,s), 5.62(1H,m), 5.96(1H,m), 7.80(2H,m), 8.05(1H,m), 10.22(1H,s) .
[0110] b) 2-Methyloxirane-2-carboxylic acid (3-methyl...
Embodiment 2
[0117] 3-(4-Acetamido-3-fluorophenoxy)-2-hydroxy-2-methyl-N-(3-methyl-4-nitrophenyl)-propionamide a}N-(2- Fluoro-4-hydroxyphenyl)acetamide
[0118] Acetic anhydride (1.3ml, 13.8mmol) was added dropwise to a solution of 4-amino-3-fluorophenol (1.0g, 7.9mmol) in acetic acid (25ml) at room temperature. The reaction mixture was stirred at room temperature for 2 hours, water (2ml) was added and stirring was continued at room temperature for 30 minutes. The mixture was evaporated in vacuo. Crude yield 1.3 g (100%) was used without further purification.
[0119] 1 H NMR (DMSO-d 6 ): 2.00 (3H, s), 6.50-6.68 (2H, m), 7.39 (1H, m), 9.39 (1H, s), 9.72 (1H, s).
[0120] b) 3-(4-acetylamino-3-fluorophenoxy)-2-hydroxy-2-methyl-N-(3-methyl-4-nitrophenyl)-propionamide
[0121] This compound was synthesized following the procedure described in Example 1c. With N-(2-fluoro-4-hydroxyphenyl)acetamide (0.5g, 3.0mmol) and 2-methyloxirane-2-carboxylic acid (3-methyl-4-nitrophenyl)amide (0.6 ...
Embodiment 3
[0123] Example 3. (Method B)
[0124] (2S)-3-(4-Acetamidophenoxy)-2-hydroxy-2-methyl-N-(3-methyl-4-nitrophenyl)-propionamide
[0125] a) (2R)-1-(2-methacryloyl)pyrrolidine-2-carboxylic acid
[0126] D-proline (5 g, 43.4 mmol) was dissolved in 2M NaOH (26 ml) and cooled in an ice bath, and the solution was diluted with acetone (26 ml). A solution of methacryloyl chloride (6.3ml, 65.1mmol) in acetone (26ml) and 2M NaOH solution (34ml) were added simultaneously to the solution of D-proline over 1 hour. After the addition was complete, the resulting mixture was stirred at room temperature for 3 hours. The mixture was evaporated at 40°C, extracted with diethyl ether (2 x 40ml) and acidified to pH 2 with concentrated HCl. The resulting mixture was extracted with ethyl acetate (3×50ml), washed with Na 2 SO 4 Dry and evaporate. Yield 11.5 g (8.0 g theoretical), the crude was used without further purification.
[0127] b) (3R,8aR)-3-bromomethyl-3-methyltetrahydropyrrolo[2,1-c][1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com