Production method of erigeron breviscopus element drop
A technology for breviscapine and a production method, which is applied in the directions of pill delivery, pharmaceutical formulations, cardiovascular system diseases, etc., can solve the problems of increasing interfacial tension, increasing contact area, small interfacial tension, etc., and achieves controlling the roundness of pellets. The effect of improving the quality of the product, controlling the difference in the weight of the pill, and improving the rate of qualified products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
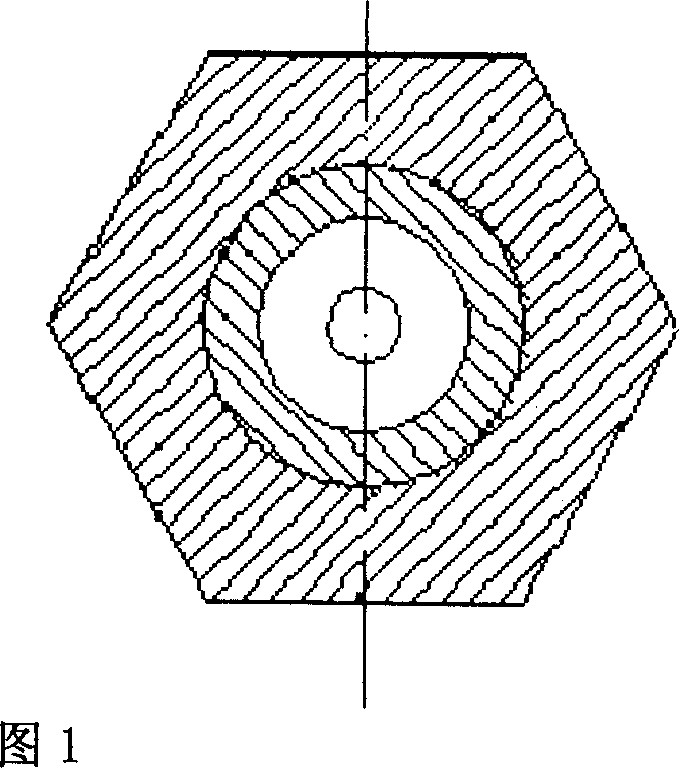
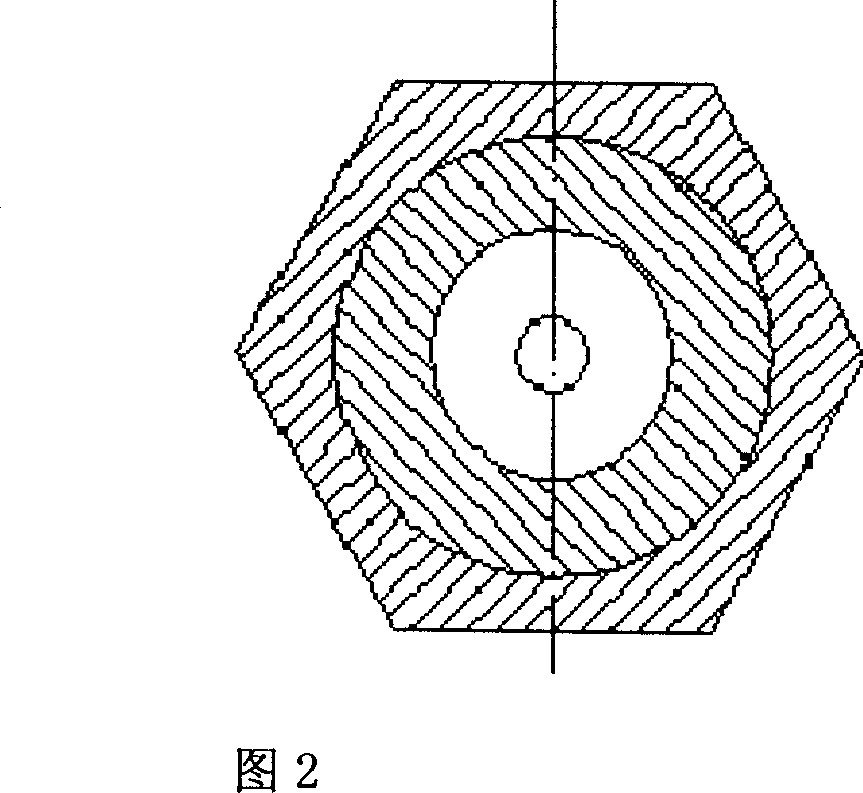
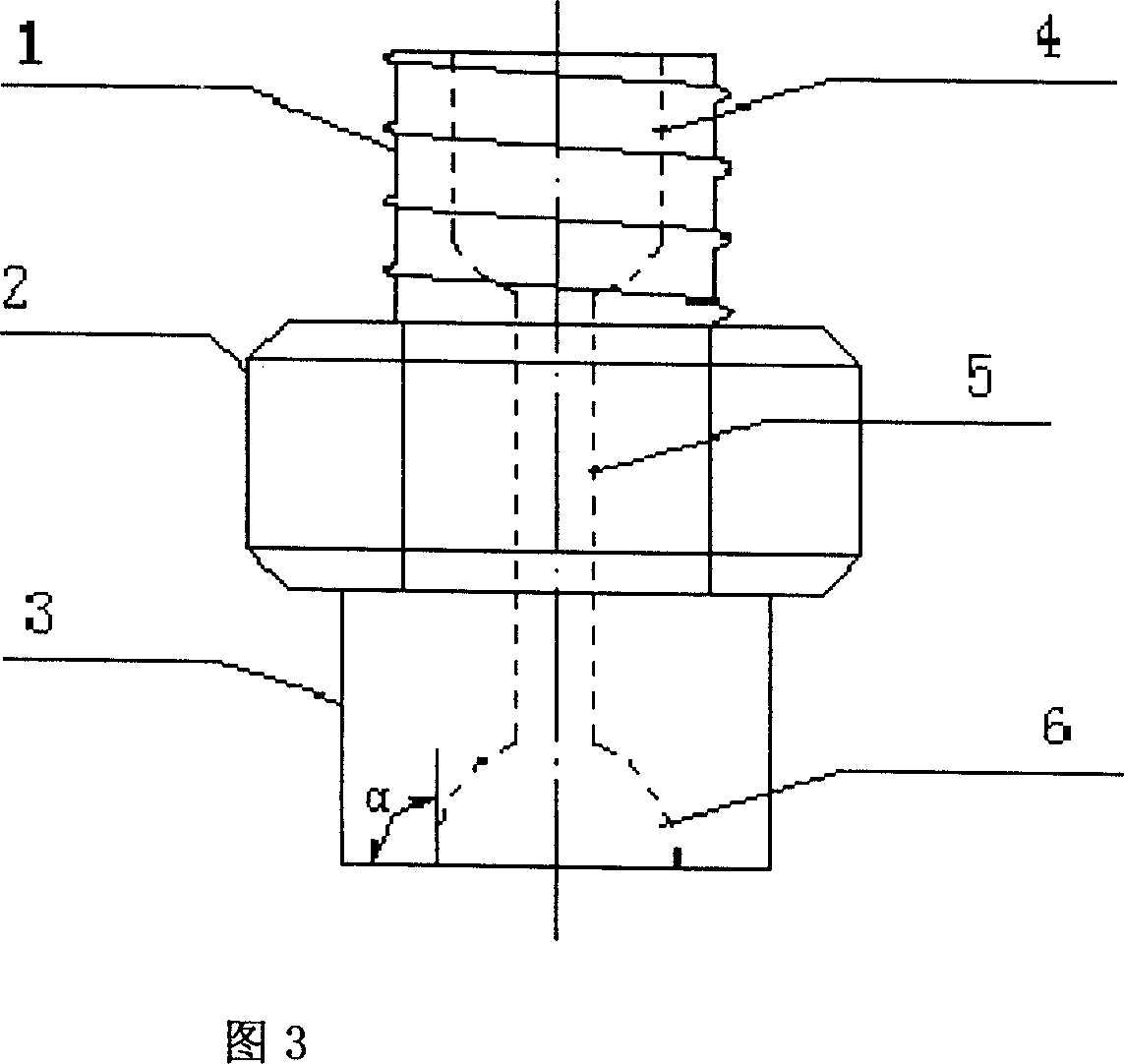
[0023] Take 1 part by weight of scutellarin fine powder crushed through a 100-mesh sieve, add it to the matrix where 1-4 parts by weight of polyethylene glycol 6000 and 2-5 parts by weight of polyethylene glycol 4000 are mixed and melted, stir well, and make a uniform The medicinal liquid is prepared by dripping with a diameter-limited cylindrical concave nozzle dropper under the condition of 75°C-85°C heat preservation, and cooled with 40°C-0°C dimethyl silicone oil, and the oil pellets are separated. Dry for 60 minutes under the conditions of 40°C and 55% relative humidity, sieve and select pellets, test, and pack to get final product.
[0024] Compared with the cylindrical plane nozzle dripper, the difference in pellet weight and qualified product rate of pellet roundness produced by the invention are increased by 30%. The dissolution rate of the product of the present invention is 95.3%.
Embodiment 2
[0026] Take 1 part by weight of breviscapine fine powder crushed through a 100-mesh sieve and add it to 2-6 parts by weight of molten polyethylene glycol 6000 matrix, stir well to make a uniform medicinal solution, and heat it at 80°C-90°C Next, use the diameter-limited cylindrical concave nozzle dropper method to drip, use 35°C to 5°C dimethyl silicone oil to gradually cool, and separate the oil pellets, under the conditions of a temperature of 45°C and a relative humidity of 50%. Dry for 30 minutes, sieve the pills to select the pills, inspect and pack, and the product is obtained.
[0027] Compared with the cylindrical planar nozzle dripper, the difference in pellet weight and qualified product rate of pellet roundness produced by the invention are increased by 21%. The dissolution rate of the product of the present invention is 96.1%.
Embodiment 3
[0029] Take 1 part by weight of scutellarin fine powder crushed through a 100-mesh sieve and add to 1-5 parts by weight of polyethylene glycol 6000, 2-4 parts by weight of polyethylene glycol 4000 and 0.1-3 parts by weight of sodium lauryl sulfate Mix and melt the matrix, stir well to make a uniform drug solution, drop it with a diameter-limited cylindrical concave nozzle dropper dripping method under the condition of 75 ° C ~ 90 ° C heat preservation, use 40 ° C ~ 5 ° C dimethicone The base silicone oil is gradually cooled, the oil pellets are separated, dried for 60 minutes at a temperature of 40°C and a relative humidity of 60%, the pellets are sieved, inspected, and packaged separately to obtain the product.
[0030] Compared with the cylindrical planar nozzle dripper, the difference in pellet weight and qualified product rate of pellet roundness produced by the invention are increased by 35%. The dissolution rate of the product of the present invention is 98.4%.
[0031]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com