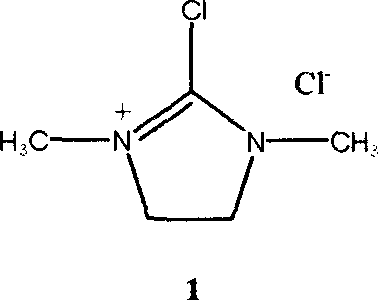
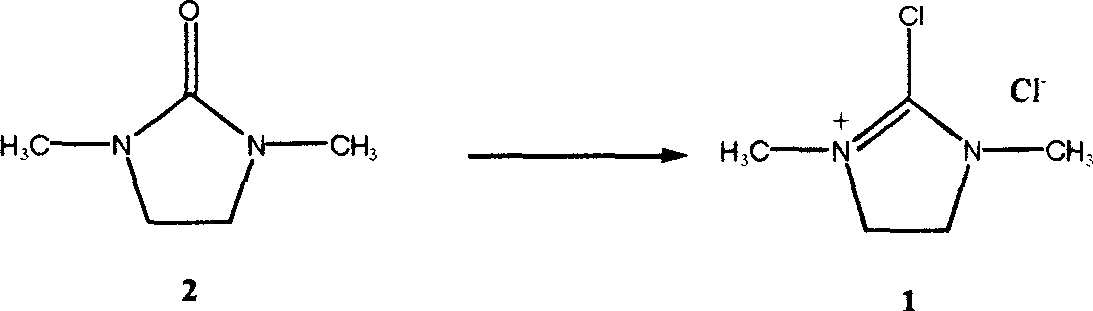
Synthesis technology of 1,3-dimethyl-2-chloroimidazoline chloride
A chloroimidazoline and synthesis process technology, applied in the field of chlorination, can solve the problems that the product is difficult to meet the requirements of pharmaceutical and chemical production, the reaction is difficult to carry out fully, the transportation and use are dangerous, and the like, so as to avoid side reactions and reduce waste gas treatment. Process, the effect of accurate weighing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 1000ml three-necked reaction flask, add 1,3-dimethyl-2-imidazolidinone (34.2 g, 0.3 mol), carbon tetrachloride (400 ml), and slowly add solid phosgene (bistrichloro Methyl carbonate) in carbon tetrachloride solution (containing 30 grams of solid phosgene, 0.1 moles, and 100 milliliters of carbon tetrachloride), the reaction mixture was kept below 5°C, vigorously stirred for 0.5 hours, and reacted at room temperature for 1 hour, The temperature was raised to 50° C. and maintained for 4 hours. After the reaction product is cooled to room temperature, filter and wash with a small amount of carbon tetrachloride to obtain a white crystalline product, which is dried in a desiccator with phosphorus pentoxide to obtain a pure white crystalline product chloro-1,3-dimethyl Base-2-chloroimidazoline 49 grams, yield 96.6%.
[0029] Melting point: 85~86℃
[0030] IR(KBr): γ=1632, 1541, 1415, 1344, 1301, 1230, 1142, 960cm -1
[0031] 1 H-NMR (CDCl 3 ): δ=3.36(s, 6H, CH 3 ×...
Embodiment 2
[0033] In a 1000ml three-necked reaction flask, add 1,3-dimethyl-2-imidazolidinone (34.2 g, 0.3 mol), methylene chloride (400 ml), and slowly add a dichloromethane solution of solid phosgene dropwise under stirring (containing 30 g of solid phosgene, 0.1 mole, and 100 ml of dichloromethane), the reaction mixture was kept below 5° C., stirred vigorously for 0.5 hours, and after 0.5 hours of reaction at room temperature, the temperature was raised to 40° C. and kept for 4 hours. The reaction product was cooled to room temperature, filtered, and washed with a small amount of carbon tetrachloride to obtain a white crystalline product, which was dried in a desiccator with phosphorus pentoxide to obtain 48 grams of a pure white crystalline product with a yield of 95%. Melting point, IR and 1 The H-NMR absorption conditions are consistent with Example 1.
Embodiment 3
[0035] In a 500ml three-necked reaction flask, add 1,3-dimethyl-2-imidazolidinone (34.2 grams, 0.3 moles), chlorobenzene (350 milliliters), and slowly add a chlorobenzene solution of solid phosgene (containing 30 grams of solid phosgene, 0.1 moles, 50 ml of chlorobenzene), the reaction mixture was kept below 5°C, vigorously stirred for 0.5 hours, and after 2 hours of reaction at room temperature, the temperature was raised to 60°C and kept for 3 hours. The reaction product was cooled to room temperature, filtered and washed with a small amount of dichloromethane to obtain a white crystalline product, which was dried in a desiccator with phosphorus pentoxide to obtain 41.5 g of a pure white crystalline product with a yield of 82%.
[0036] Melting point, IR and 1 The H-NMR absorption conditions are consistent with Example 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap