Methods for the treatment of tinnitus induced by cochlear excitotoxicity
An excitatory and cochlear technology, applied in pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., can solve problems such as unreliable prediction of tinnitus and unclear molecular mechanism.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Methods and materials
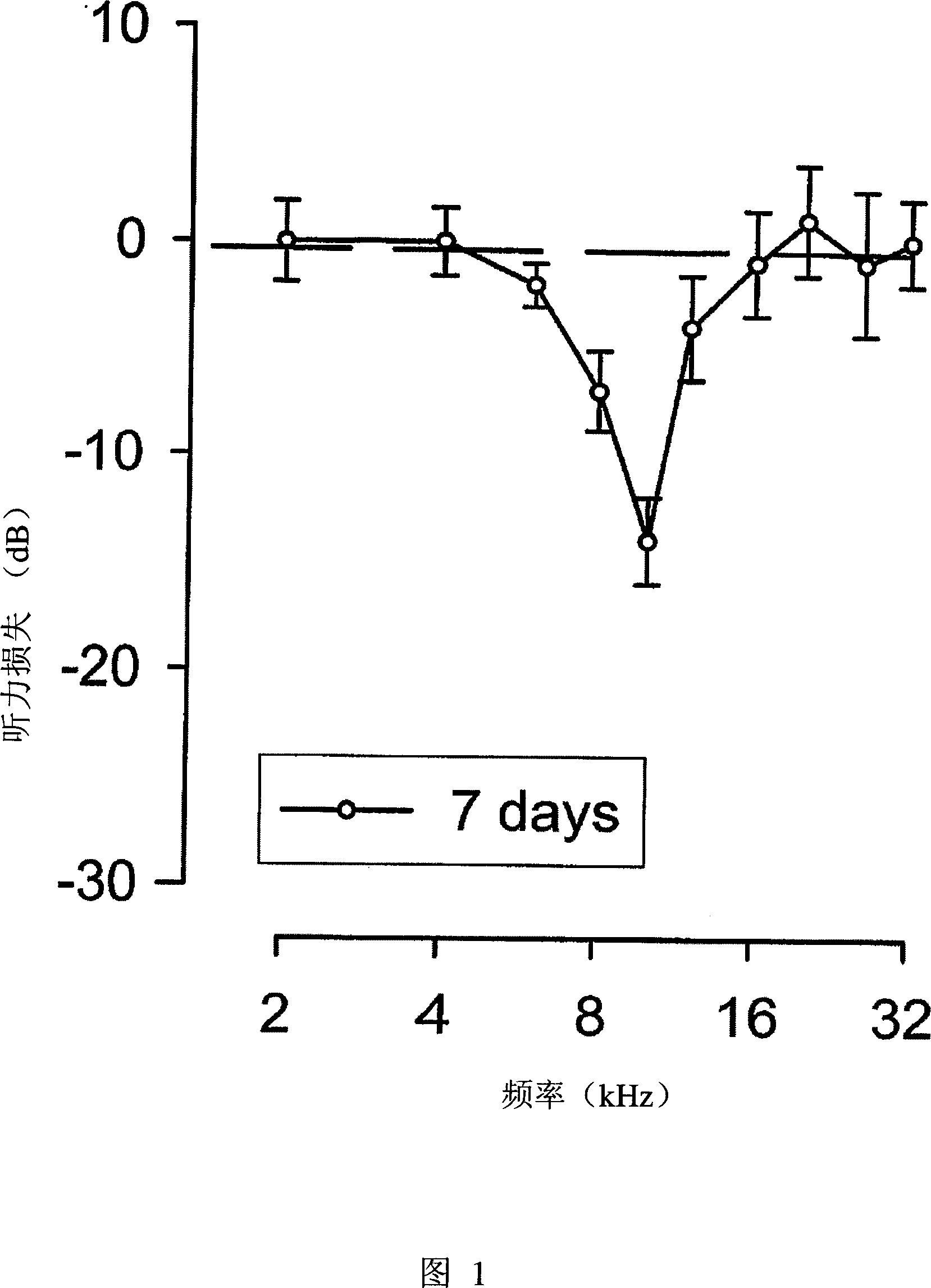
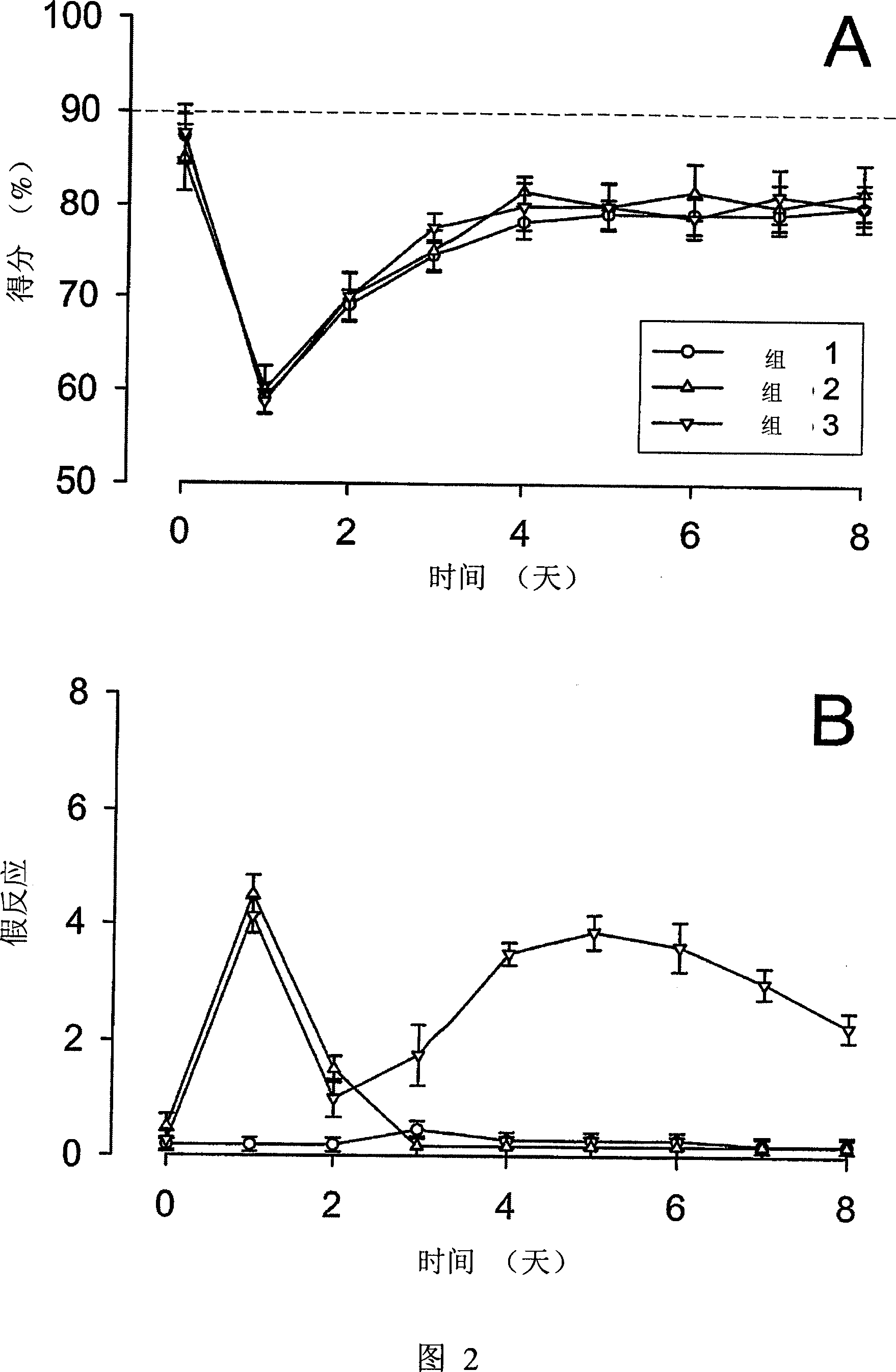
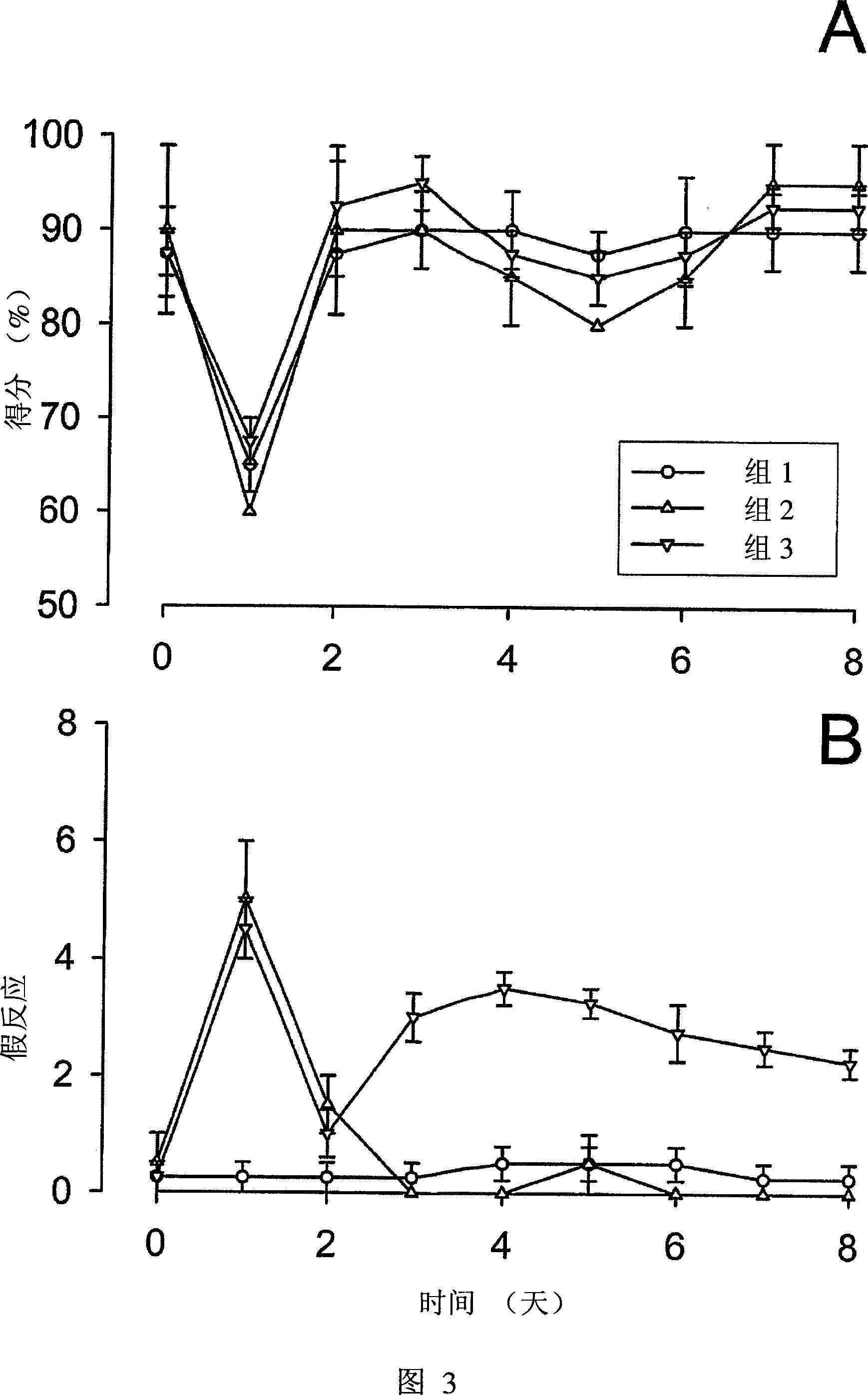
[0059] We study and test animal models of tinnitus caused by ototoxic excitotoxicity, which is caused by hearing damage. In general, tinnitus cannot be directly observed, because not all individuals’ hearing impairments cause tinnitus, and the sensation of tinnitus can be maintained for a few hours after the occurrence of excitotoxicity, or forever, this animal model The definition and implementation of this is a big challenge. For example, these things to consider include the need for more animals to obtain a sufficient number of tinnitus cases for research, and allowing tinnitus to be observed for a period of time. Because it is not clear whether tinnitus cases caused by cochlear excitotoxicity will continue, it is recommended to conduct research in the early stages of tinnitus.
[0060]The experiment was carried out in two stages. First, in the case of applying the therapeutic compound, the hearing loss after acute hearing loss and the appearance ...
Embodiment 2
[0086] Methods and materials
[0087] In order to evaluate the different mechanisms of tinnitus caused by salicylate and excitotoxicity, after the induction of these two different types of tinnitus, a comparative morphological analysis of the cochlear sensory nerve and Western Blot immunodetection (Western Blot immunodetection) were performed.
[0088] morphology
[0089] Two groups of Long Evans mice were used, 3 in each group. One group was intraperitoneally injected with a 350 mg / kg salicylic acid solution twice a day for 2 days; as in Example 1, the hearing of the other group was damaged. After the head of the deeply anesthetized rat was removed, the cochlea was removed from the temporal bone, and the cochlea was perfused with a fixative solution, which was 0.1M phosphate buffered saline (PBS), 2.5% glutaraldehyde, and pH 7.3. The cochlea is then processed, followed by scanning (SEM) processing or transmission electron microscopy (TEM) processing. For SEM, the auditory cartila...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com