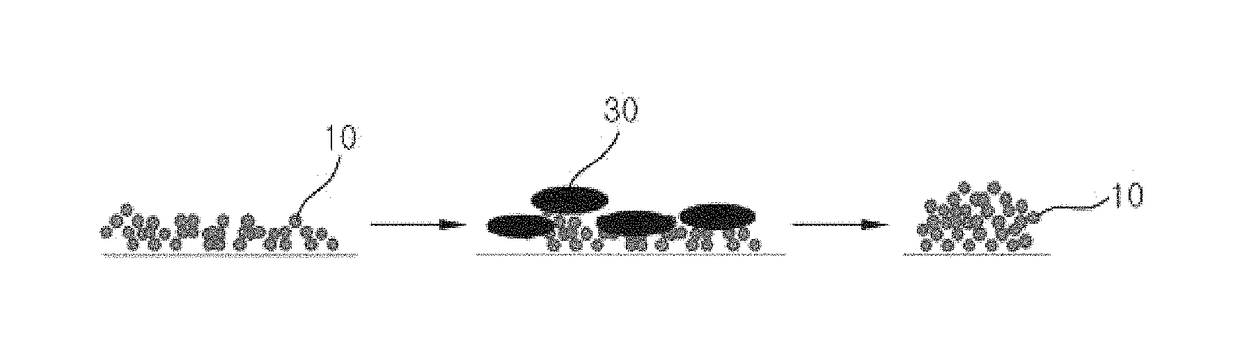
Thermally stable monolith catalyst for reforming reaction
a monolith catalyst, thermal stability technology, applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, sustainable manufacturing/processing, etc., can solve the problem of increasing the burden on the industry, deteriorating the catalytic activity, and reducing the activation point, so as to reduce the amount of catalyst used.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example
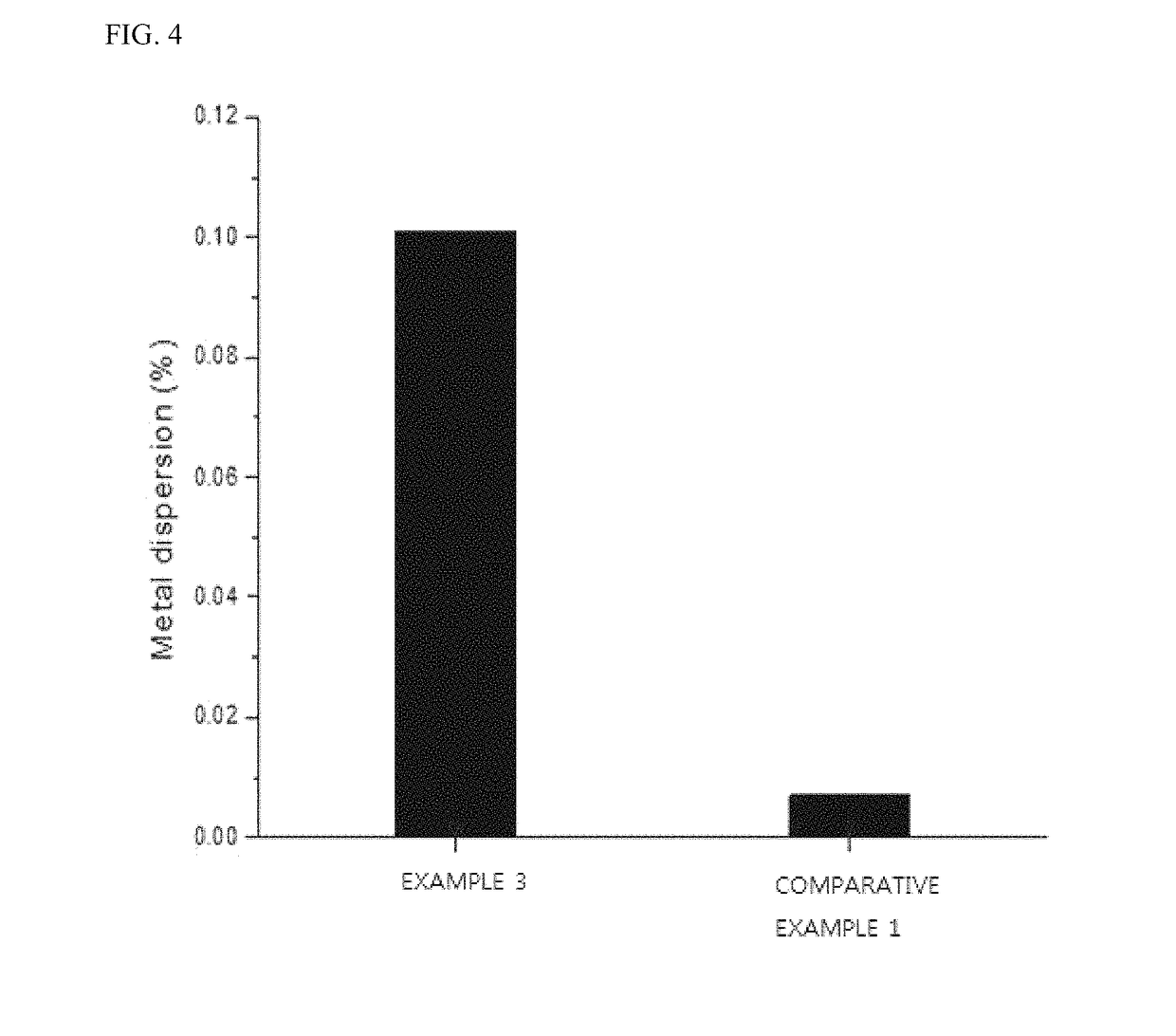
[0061]In order to assess stability against degradation of the monolith catalyst, an extent of sintering the catalytic active material was determined through CO chemisorption after performing heat treatment at 1000° C. for 24 hours. As shown in the following Table 1, in a case of the monolith catalyst including Al added as a barrier, it could be seen that CO adsorption quantity indicating the number of activation points is 5 to 12 times higher than the monolith catalyst without Al addition (Comparative Example 1). Furthermore, a dispersion rate of active ingredients was also found to be 6 to 14 times higher than Comparative Example 1.
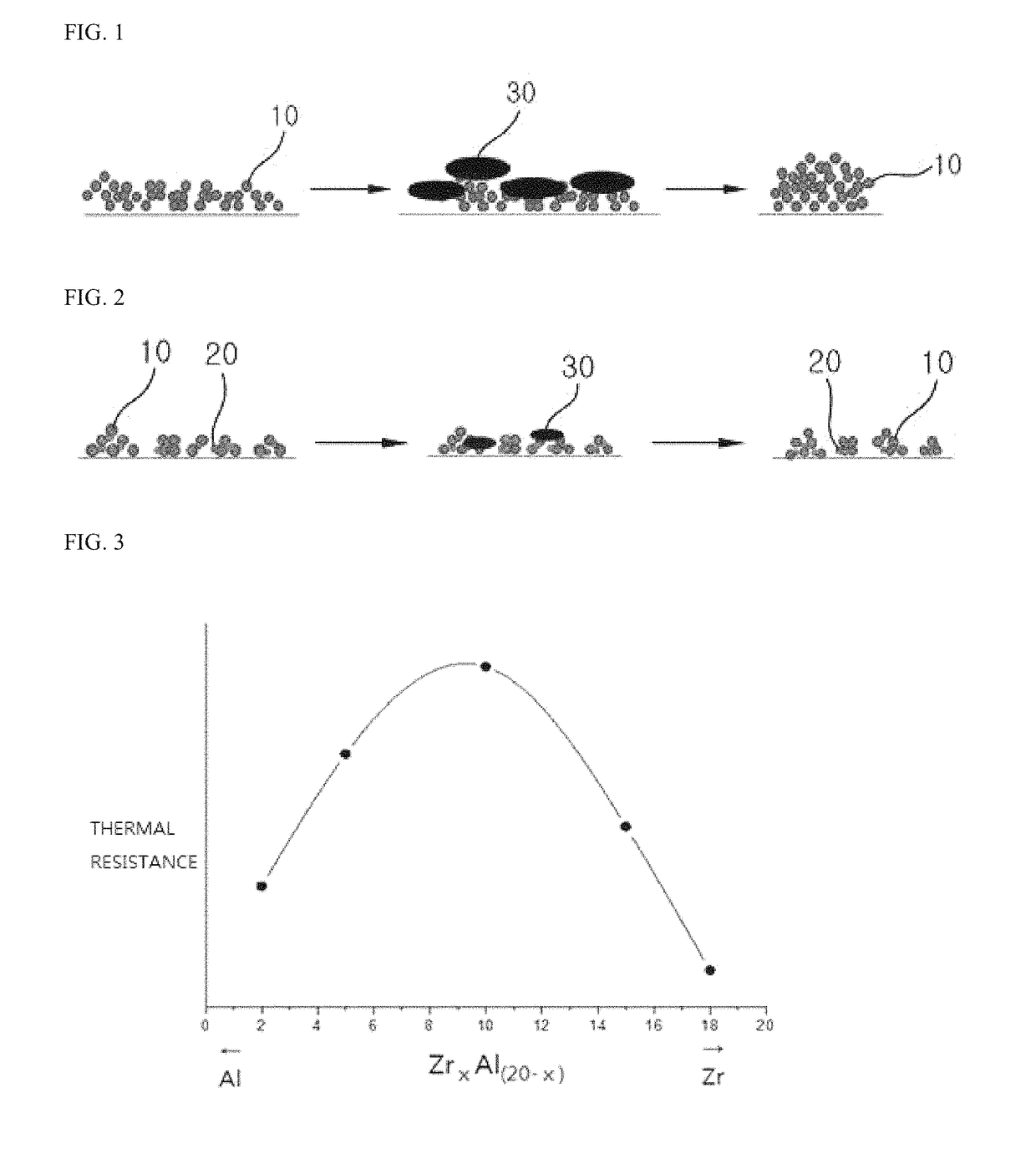
[0062]In particular, at a molar ratio of Zr to Al of 1:1, the largest CO adsorption quantity and the highest metal dispersion rate were observed, thereby indicating that, due to the degradation, the most stable addition ratio of the barrier may be denoted by Zr:Al=1:1. This result could be demonstrated by the graph of FIG. 3 illustrating a change in ther...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com