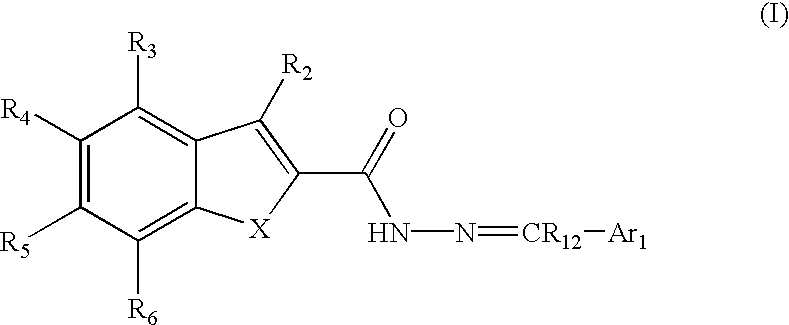
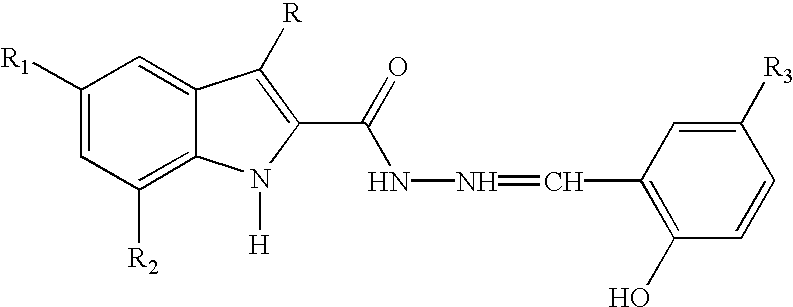
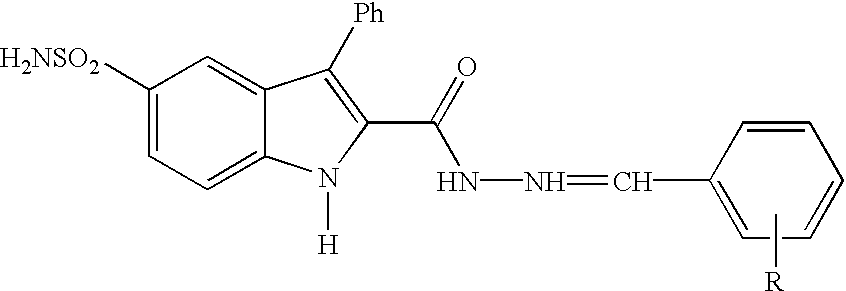
Substituted indole-2-carboxylic acid benzylidene-hydrazides and analogs as activators of caspases and inducers of apoptosis and the use thereof
a technology of benzylidenehydrazide and indole-2-carboxylic acid, which is applied in the field of substituted indole-2-carboxylic acid benzylidenehydrazide and analogs, can solve the problems of bone marrow toxicity, cancer cells lose the capacity to undergo cellular suicide, and cancer cells become cancerous, so as to prevent or ameliorate neoplasia and cancer. , to achieve th
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
5-Chloro-3-methyl-indole-2-carboxylic Acid (4-Methylbenzylidene)-hydrazide
[0188] A solution of 5-chloro-3-methyl-indole-2-carboxylic acid hydrazide (13 mg, 0.054 mmol), p-tolualdehyde (6.5 mg, 0.054 mmol) in ethanol (5 mL) was refluxed for 8 h. It was evaporated in vacuo and the residue was purified by column chromatography (silica gel, EtOAc / Hexane=4:1) to give 7.8 mg (42%) of the title compound. .sup.1H NMR (CD.sub.3OD): 8.26 (s, 1H), 7.72 (d, J=8.1 Hz, 2H), 7.62 (bs, 1H), 7.39 (d, J=8.7 Hz, 1H), 7.28-7.21 (m, 3H), 2.58 (s, 3H), 2.39 (s, 3H).
example 2
5-Chloro-3-methyl-indole-2-carboxylic Acid (Benzylidene)-hydrazide
[0189] The title compound was prepared similar to Example 1. From 5-chloro-3-methyl-indole-2-carboxylic acid hydrazide (26 mg, 0.11 mmol) and benzaldehyde (11 mg, 0.11 mmol) was obtained 14 mg (39%) of the title compound. .sup.1H NMR (DMSO-d.sub.6): 11.56 (bs, 1H), 8.37 (bs, 1H), 7.73 (bs, 2H), 7.71 (bs, 1H), 7.55-7.44 (m, 3H), 7.23 (d, J=7.2 Hz, 1H), 4.40 (bs, 1H), 2.50 (s, 3H).
example 3
5-Chloro-3-methyl-indole-2-carboxylic Acid (4-Dimethylaminobenzylidene)-hy- drazide
[0190] The title compound was prepared similar to Example 1. From 5-chloro-3-methyl-indole-2-carboxylic acid hydrazide (24 mg, 0.1 mmol) and p-dimethylaminobenzaldehyde (14.9 mg, 0.1 mmol) was obtained 5 mg (21%) of the title compound. .sup.1H NMR (CD.sub.3OD): 10.9 (bs, 1H), 9.07 (bs, 1H), 8.2-6.6 (m, 6H), 3.06 (s, 6H), 2.70 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com