Compounds which inhibit HIV replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
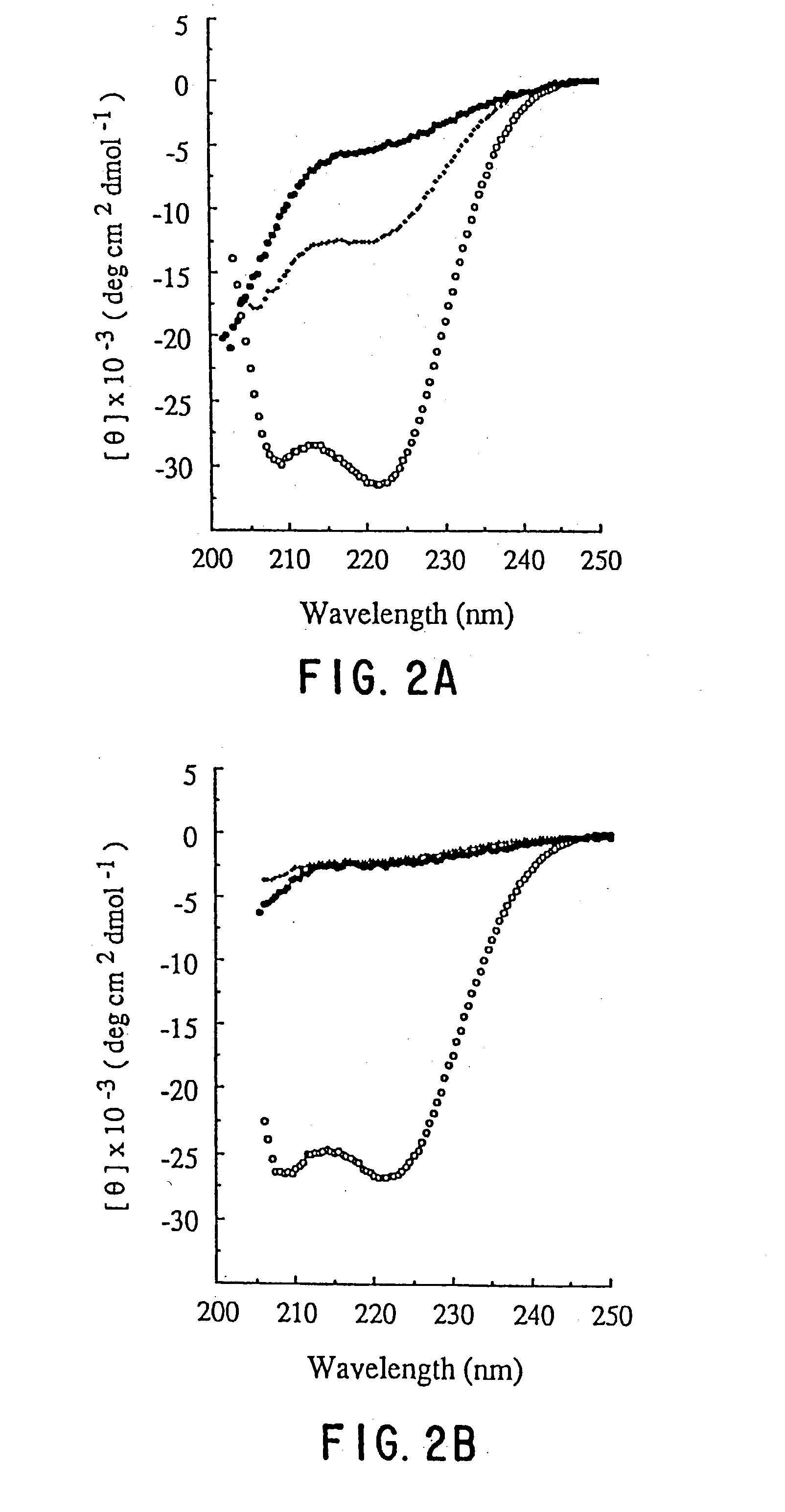
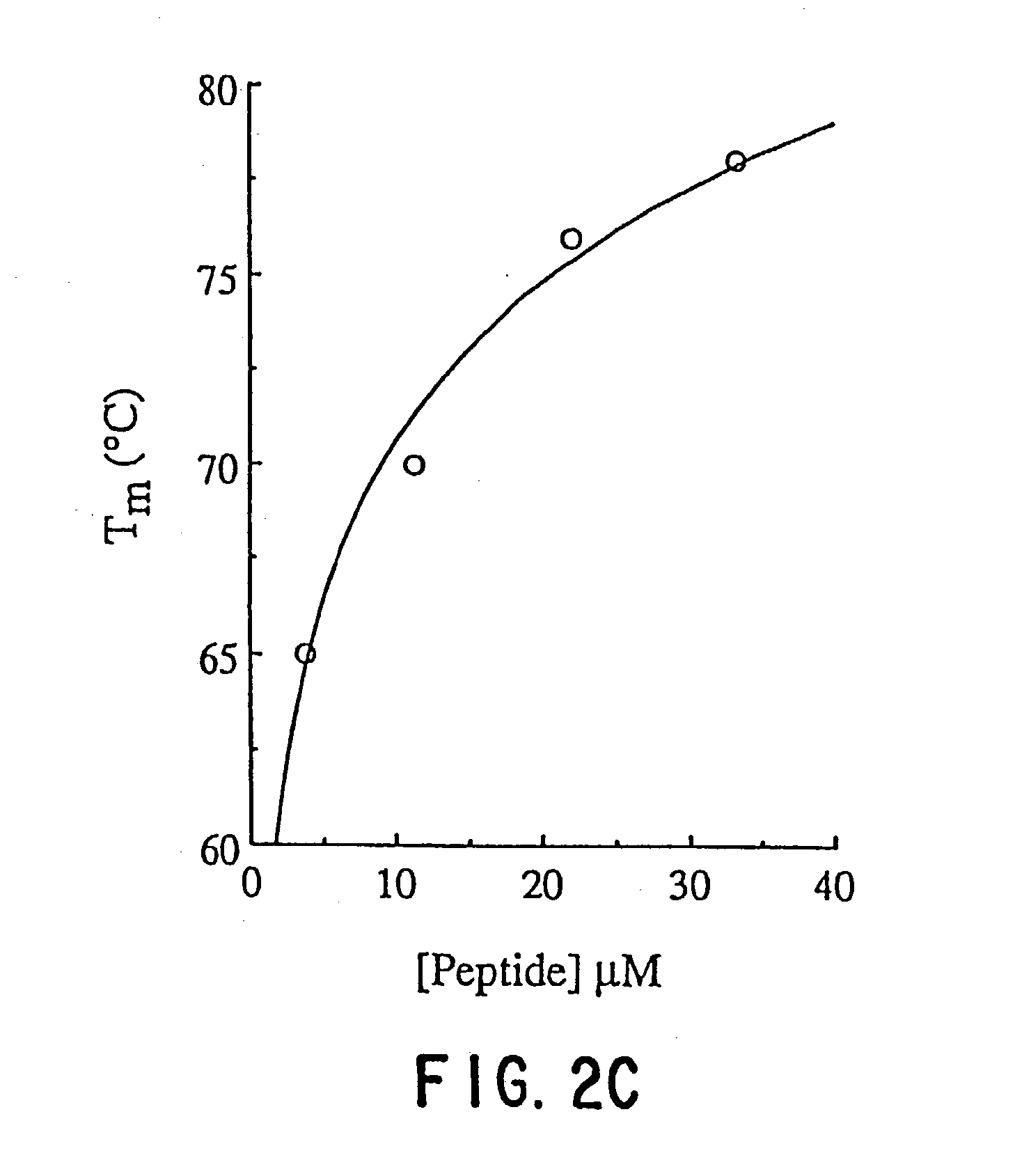
[0023] The-term "HIV" as used herein refers to HIV-1, and the numbering of amino acids in HIV proteins and fragments thereof given herein is with respect to the HIV1.sub.LAI isolate. It is to be understood, however, that while HIV viral infection and the effects of DP-107 on such HIV infection are being used herein as a model systems in which the potential anti-viral properties of peptides capable of forming coiled coils are described, such properties of coiled coil peptides may represent generalized mechanisms by which a broad spectrum of enveloped viral infections may be inhibited. Enveloped viruses whose infectivity may be inhibited using the coiled coil peptides of the invention may include, but are not limited to, other HIV strains such as HIV-2, as well as influenza viruses, syncytial respiratory viruses, an herpes viruses.
[0024] The DP107 peptide sequence is based on a highly conserved region in the transmembrane protein (TM) which was predicted by Gallaher et al., AIDS Res. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com