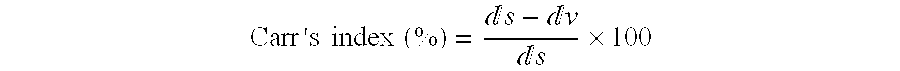Modified carrier particles for use in dry powder inhalers
a carrier particle and dry powder technology, applied in the direction of woodworking apparatus, pharmaceutical delivery mechanism, granular delivery, etc., can solve the problems of moderate reduction of the crystallinity of the additives used, hard treatment,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of a Salbutamol Base / Lactose Binary Mixture
[0067] Analogously to what described in example 1, a mixture containing micronised salbutamol base as active ingredient in a ratio of 200 .mu.g to 24 mg total mixture was prepared.
[0068] The poured and tapped densities and the flowability characteristics were determined as described in example 1. The dry powder for inhalation was loaded in a Pulvinal.RTM. inhaler and the aerosol performances were determined as described in example 1.
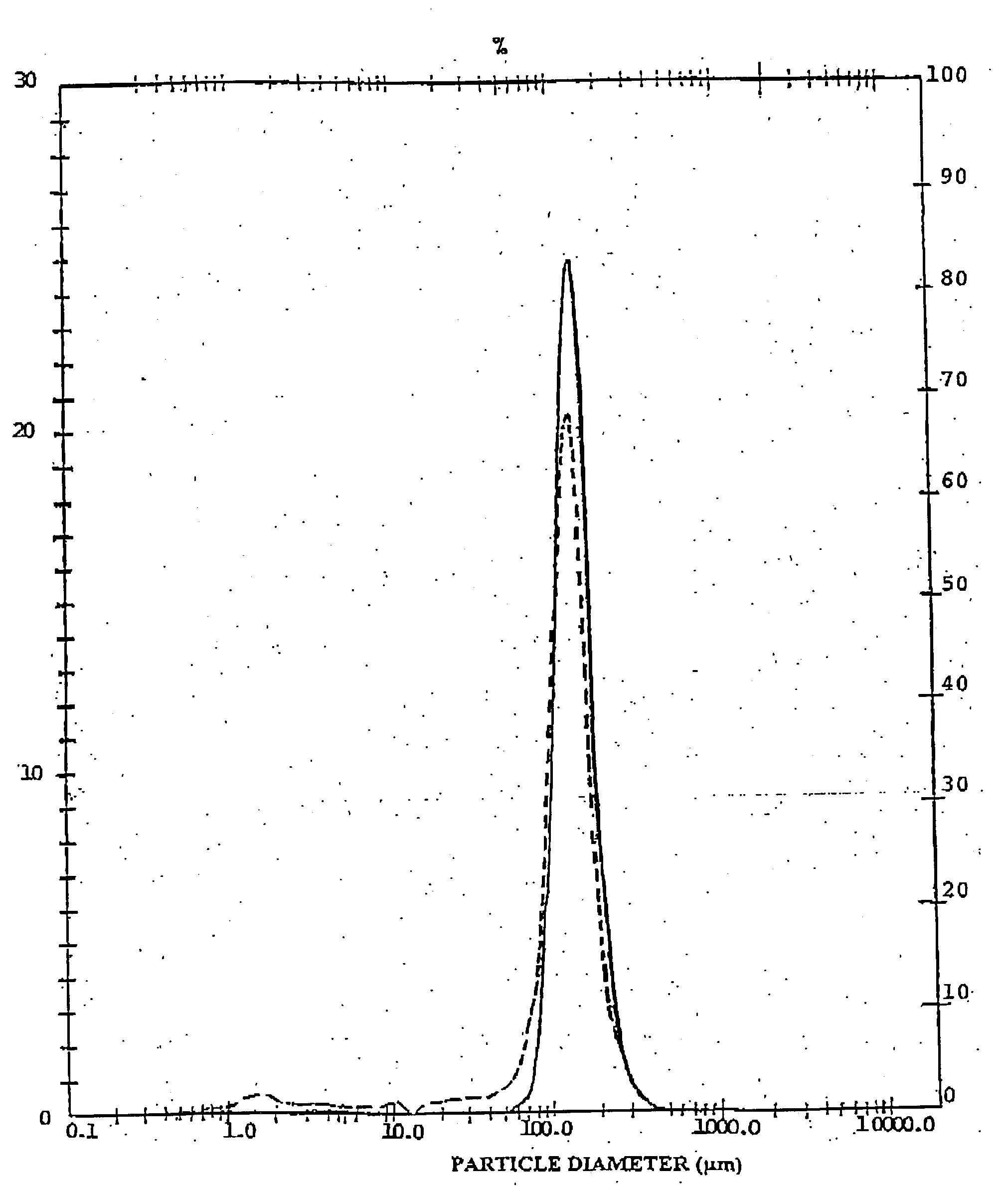
[0069] The results are reported in Table 3 in comparison with a similar preparation obtained by mixing the active ingredient with .alpha.-lactose monohydrate lactose 90-150 .mu.m not pre-treated in a mixer (standard preparation).
3TABLE 3 Preparation of Example 2 Standard preparation Technological parameters Apparent Density (g / mL) Poured 0.71 0.74 Tapped 0.78 0.83 Flodex test (.0. 4 mm) 4 4 Flow Rate through .0. 4 mm (g / min) 72 --Carr Index (%) 9 11 TSI test Mean weight (mg) 22.2 (1.7) 25.2 (3.3) Emi...
example 3
Preparation of a BDP / Lactose / Magnesium Stearate Ternary Mixture
[0072] The powder carrier was prepared according to Example 1 a) by mixing .alpha.-lactose monohydrate for 30 minutes in a sigma blade mixer. Afterwards lactose was mixed with 0.25% by weight of magnesium stearate in a Turbula mixer for two hours. Finally the dry powder for inhalation was prepared by mixing an amount of micronised beclomethasone dipropionate corresponding to a dose of 200 .mu.g and the carrier (lactose+magnesium stearate) for 30 minutes in a Turbula rotting mixer at 32 rpm.
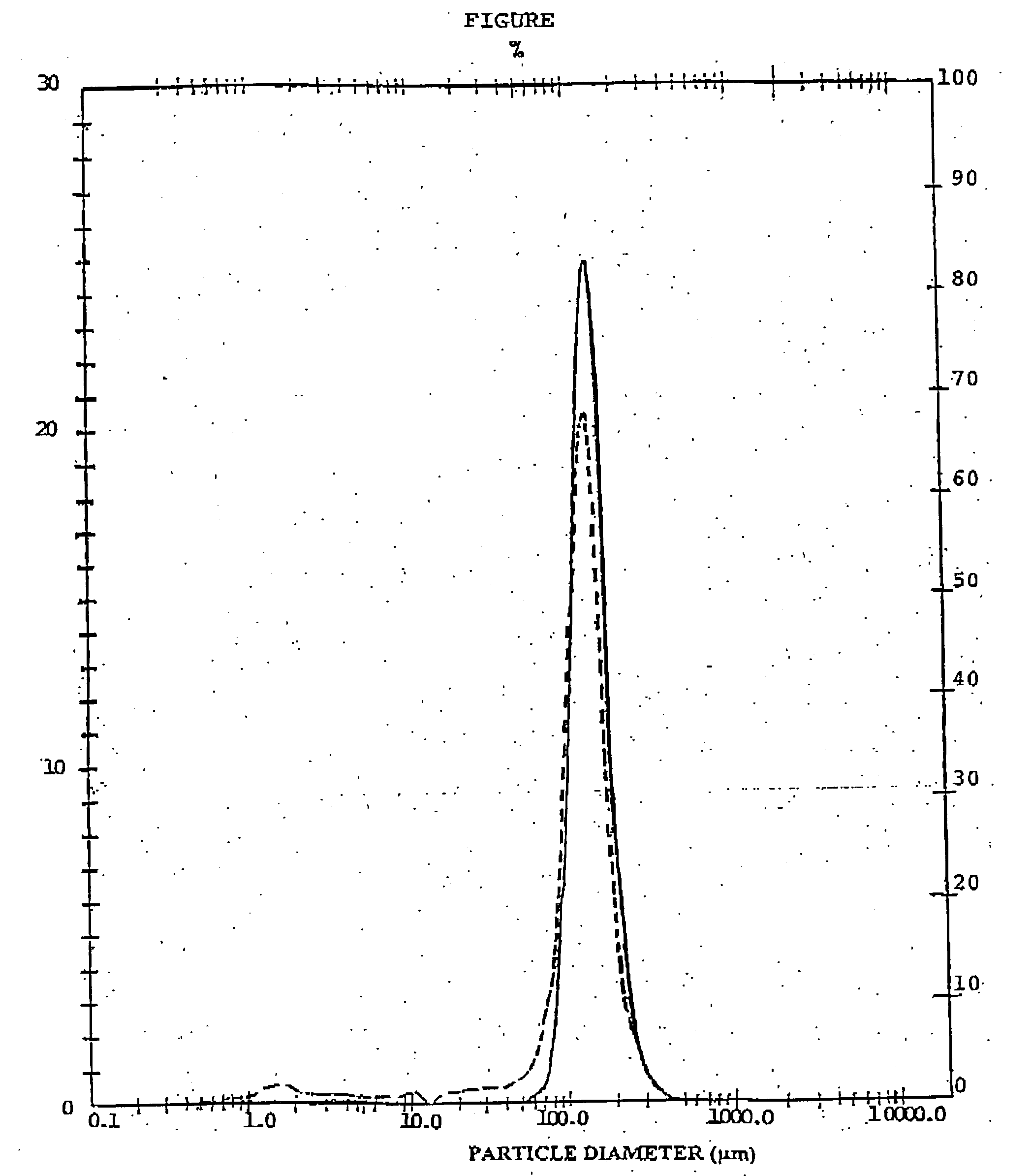
[0073] The poured and tapped densities, the flowability characteristics as well as the aerosol performances were determined as described in example 1.
[0074] The results are reported in Table 4 in comparison with a standard formulation obtained by mixing 200 .mu.g of micronised BDP with a carrier powder consisting of 99.75% by weight of .alpha.-lactose monohydrate 90-150 .mu.g not pre-treated in a mixer, and 0.25% by weight of magnesium...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean aerodynamic diameter | aaaaa | aaaaa |
| mean aerodynamic diameter | aaaaa | aaaaa |
| mean diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com