Antimicrobial agents and uses thereof
a technology of antibiotics and antibacterial agents, applied in the field of antibacterial agents, can solve the problems of increasing compromise of the benefit of antibiotic use as a means of treating infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compounds Having a 12-hydroxy-12-methylfuranone Ring
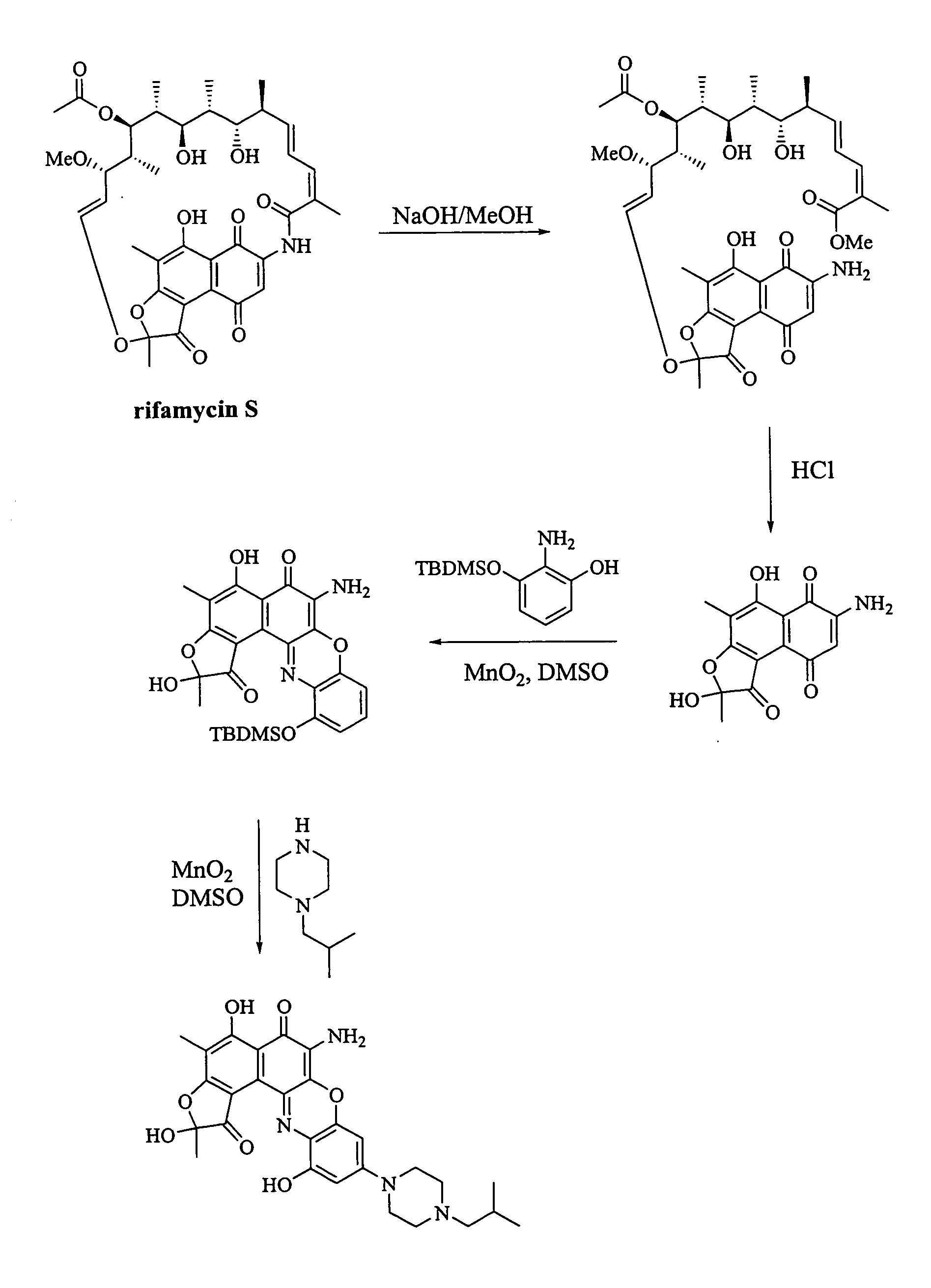
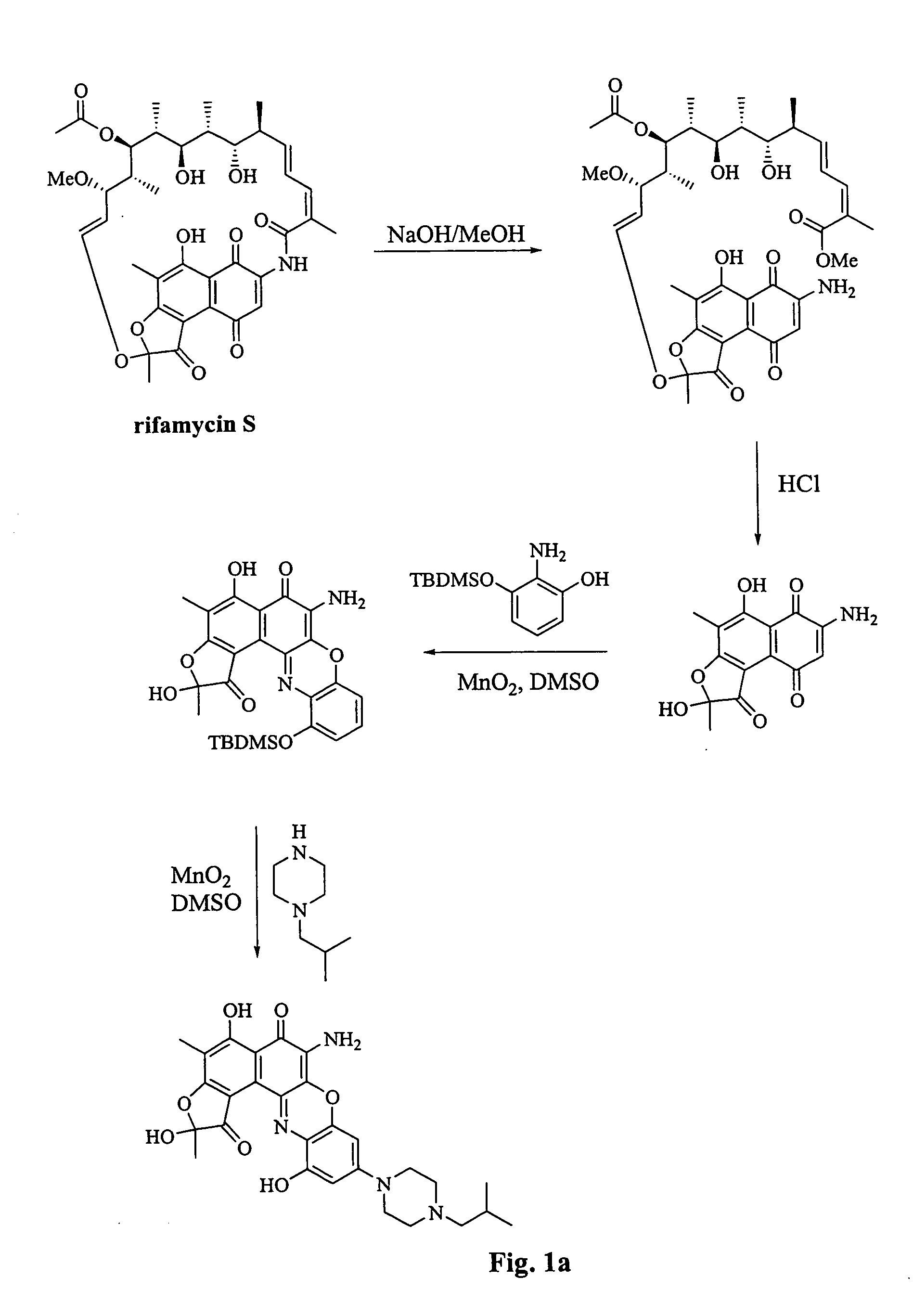
[0103] The ansa ring of Rifamycin S can be cleaved at the amide group using basic conditions as described in, for example, Bartolucci et al., Farmaco 50(9):587-593, 1995. Following amide cleavage, the ansa ring can be removed by cleavage at the dihydrofuranone group with boron triflouride, as described by Bartolucci, ibid (see FIG. 1a). The resulting product, compound X, is a useful starting material for the synthesis of compounds of formulas (I) and (II).
example 2
Preparation of Compounds Having a methyl-furan Ring
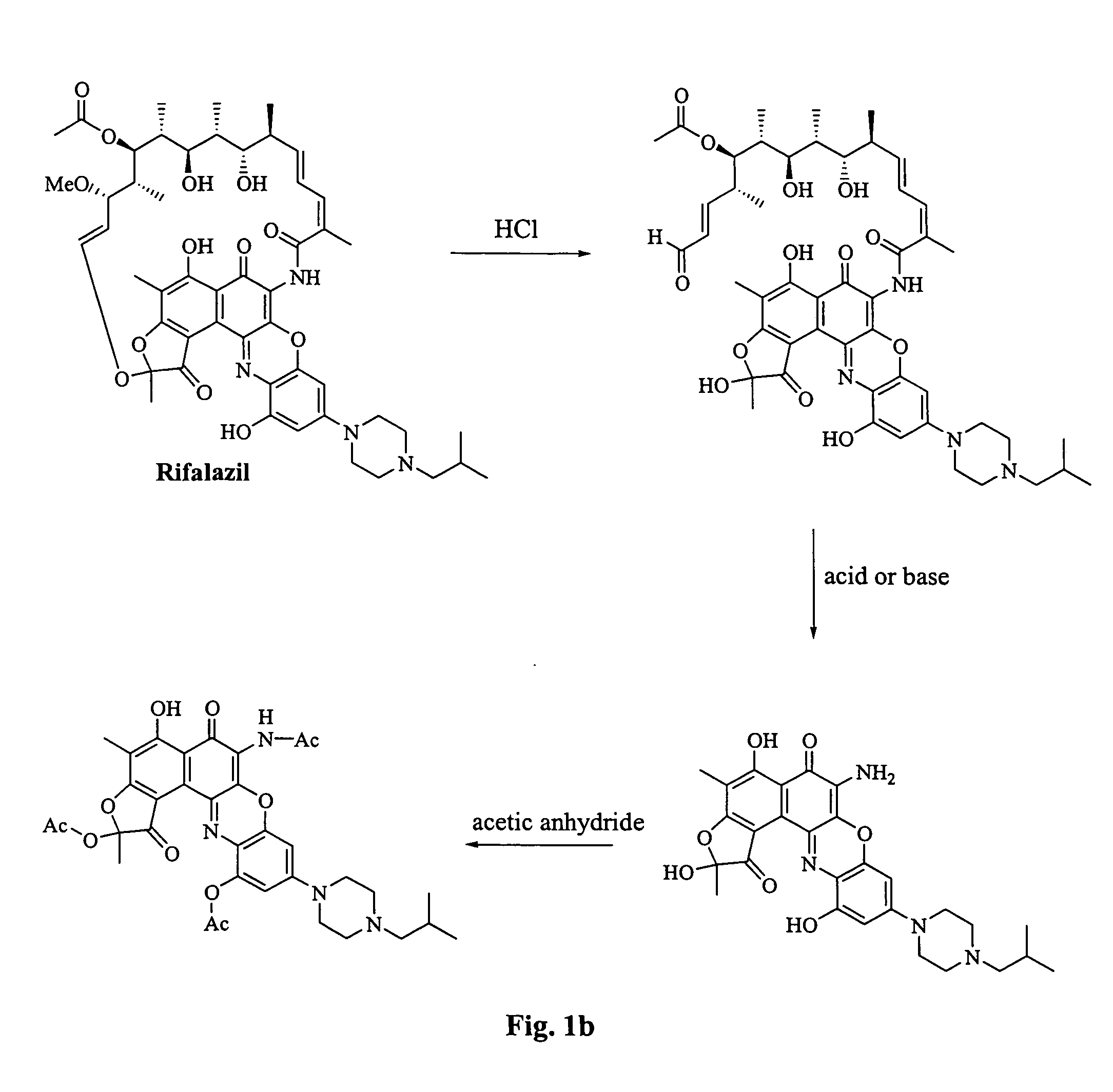
[0104] Following amide cleavage, the ansa ring of rifamycin S can also be cleaved at the the dihydrofuranone group using NaBH4, as described by Bartolucci, ibid. The resulting product, compound XI, a reduced form of compound X, is a useful starting material for the synthesis of compounds of formulas (I) and (III).
example 3
Preparation of Compounds or Formulas (I), (II), and (III)
[0105] The compounds described by formulas (II) or (III) can be synthesized using methods analogous to those disclosed in Yamane et al., U.S. Pat. No. 4,690,919; Yamane et al., U.S. Pat. No. 4,983,602; Yamashita et al., U.S. Pat. No. 5,786,349; Yamashita et al., U.S. Pat. No. 5,981,522; Kano et al., U.S. Pat. No. 4,859,661 and Chem. Pharm. Bull., 41:148, 1993, each of which is hereby incorporated by reference.
[0106] The reaction conditions previously disclosed can be used where compound X or XI is substituted for rifamycin S (see FIG. 1a).
PUM
| Property | Measurement | Unit |
|---|---|---|
| chemical structure | aaaaa | aaaaa |
| chemical structures | aaaaa | aaaaa |
| structures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com