Vaccine
a streptococcus pneumonia and vaccine technology, applied in the field of vaccines, can solve the problems of inadequate th-cells available to provide the necessary help, diminish the immune response, and immunological effects, and achieve the effects of preventing or ameliorating pneumococcal infection, eliciting a protective immune response, and preventing or ameliorating the infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
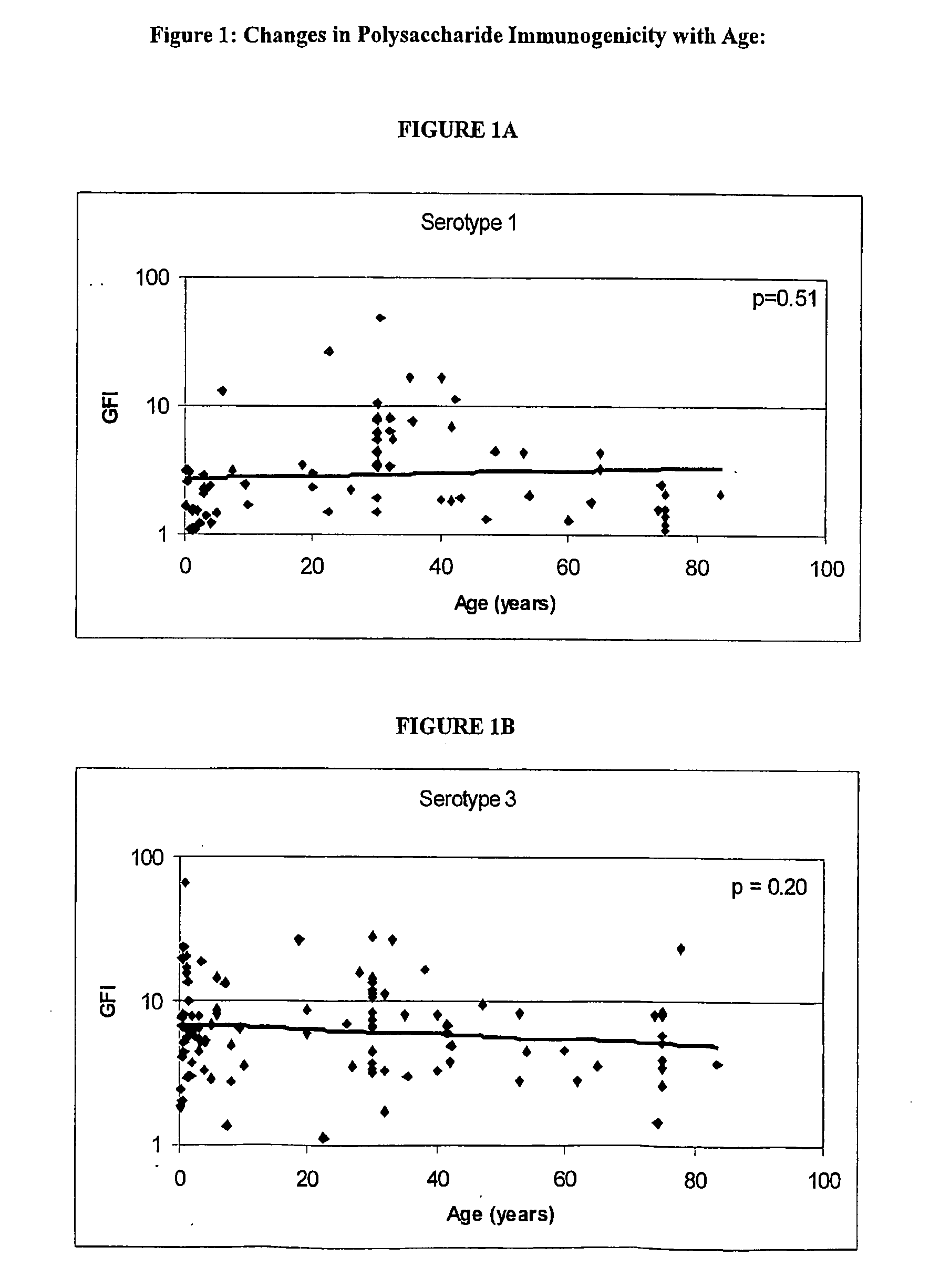
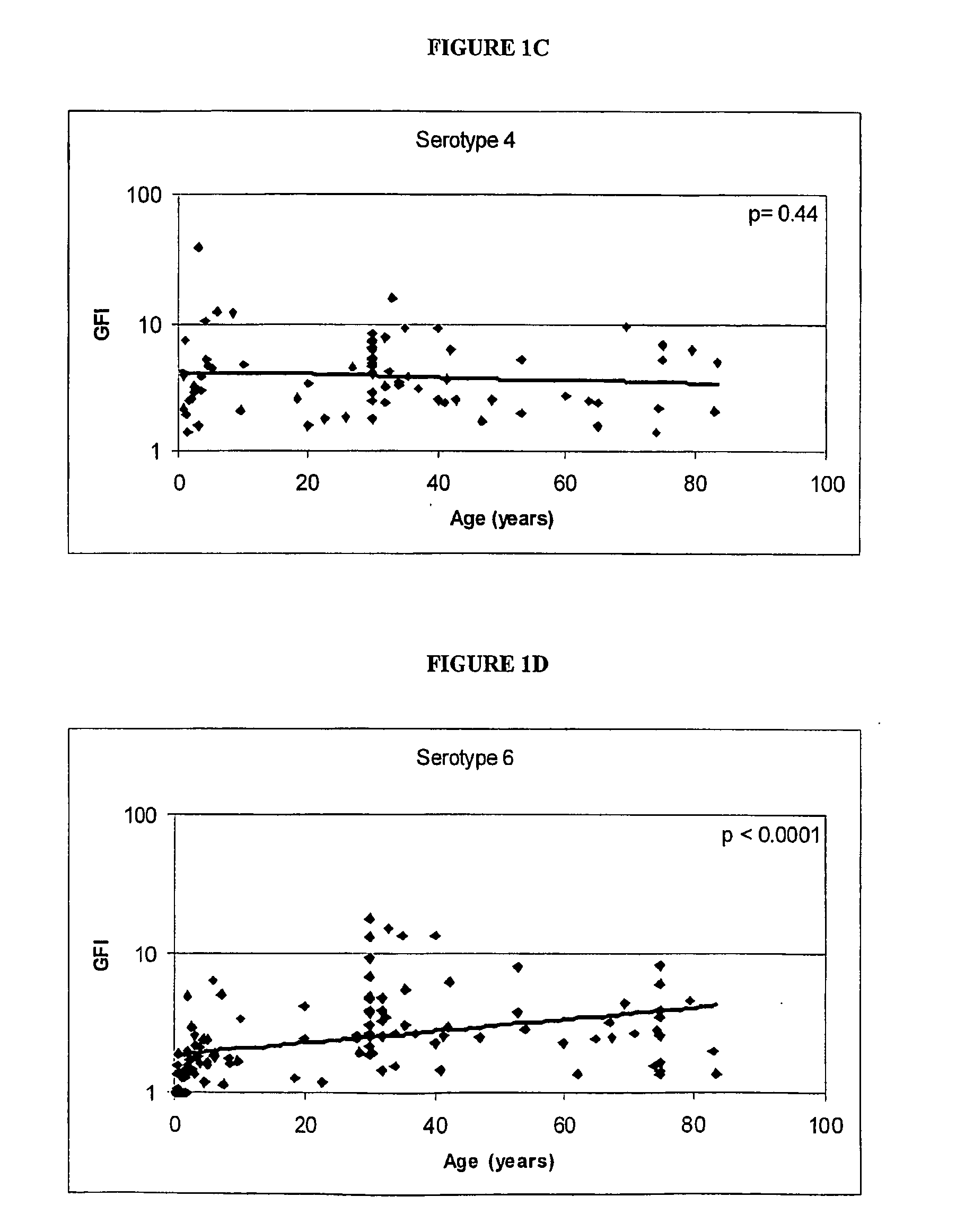
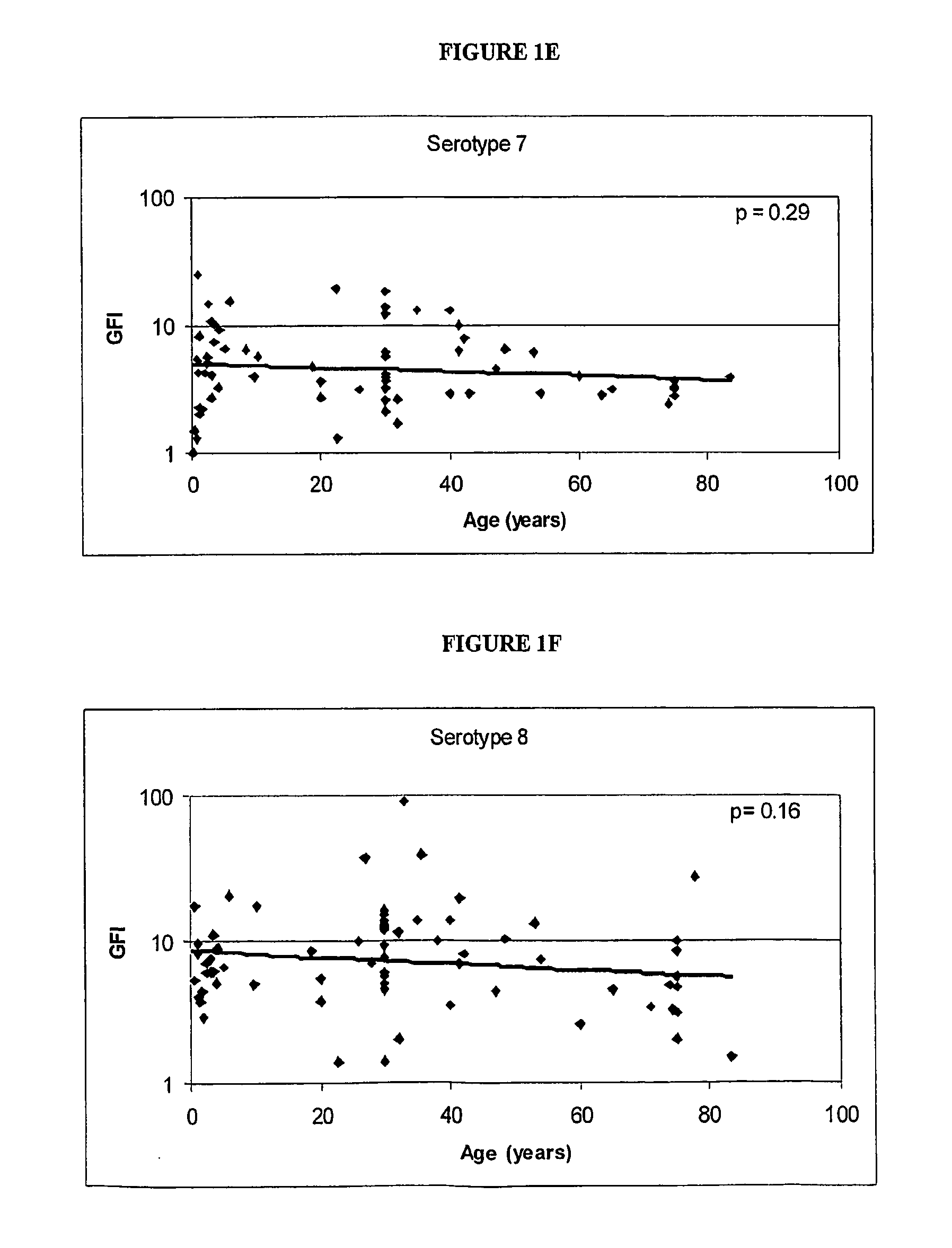
Determination of the Polysaccharides to which the Immune Response is Regulated with Age
[0062] Human antibody titers to both pre-immune and post-immunization (2 weeks to 3 months) polysaccharides (unconjugated) were collected either internally or via external sources. FIG. 1 shows the relationship between the immunogenicity of each serotype polysaccharide, as measured by the geometric mean fold-increase in antibody titre (GFI) after polysaccharide immunization, and the mean age of the subjects in the study. The linear correlations of log geometric mean fold increase and age give an indication if the immune response is regulated with age. As shown in FIG. 1, serotypes 6, 14, 19 and 23 are significantly correlated with age (por =0.20).
example 2
General Methodology of Determining Antibody Responses in Various Mammals
[0063] The sera were tested for IgG antibodies to the pneumococcal polysaccharides by an ELISA based on a consensus assay for human sera proposed by the joint CDCAWHO workshops held between 1994 and 1996 (WHO 1996, Plikatis et al J. Clin. Microbiol 38: 2043 (2000)). Briefly, purified capsular polysaccharides from ATCC (Rockville, Md., 20852) were coated at 25 μg / ml in phosphate buffered saline (PBS) on high binding microtitre plates (Nunc Maxisorp) overnight at 4 C. The plates were blocked with 10% fetal calf serum (FCS), 1 hour at 37 C. Serum samples were pre-incubated with the 20 μg / ml cell-wall polysaccharide (Statens Serum Institute, Copenhagen) and 10% FCS at room temperature for 30 minutes to neutralize antibodies to this antigen. A reference serum 89SF (courtesy of Dr. C Frasch, USFDA) was treated in the same fashion, and included on every plate. The samples were then diluted two-fold on the microplate ...
example 3
Effects of Combination of Pneumococcal PS-PD Conjugates on Immunogenicity in Adult Rats
[0076] It has been observed that the combination of vaccines into multivalent formulations can result in the decrease in immunogenicity of one or more components of the vaccine. This has been especially observed for conjugate vaccines, and has been called carrier-induced epitopic suppression. The underlying mechanism for this suppression is not well understood, but it tends to happen at higher dosages of carrier protein.
[0077] An 11-valent pneumococcal conjugate vaccine is an example of combination vaccines. Since the combination of each serotype's conjugate will add to the total amount of protein used to immunize, it is important to determine whether the combination of each conjugate vaccine into a multivalent formulation results in a significant decrease in the immunogenicity of the conjugate.
Protocol:
[0078] Adult rats were immunized with pneumococcal-polysaccharide protein D conjugate vac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com