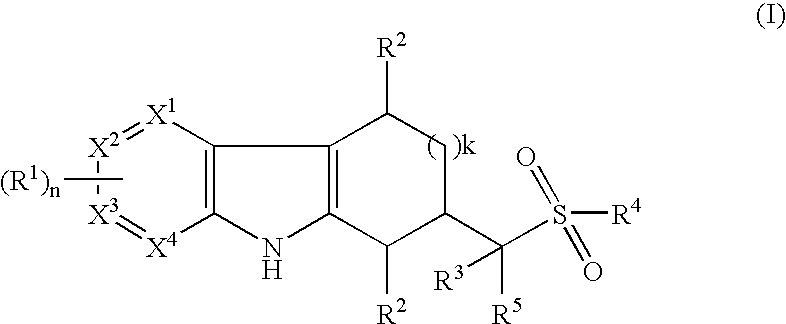
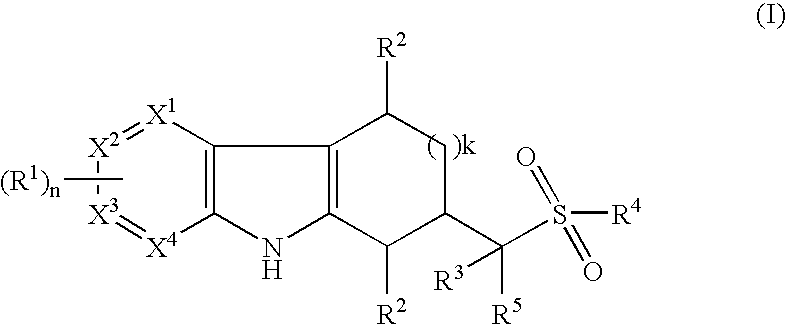
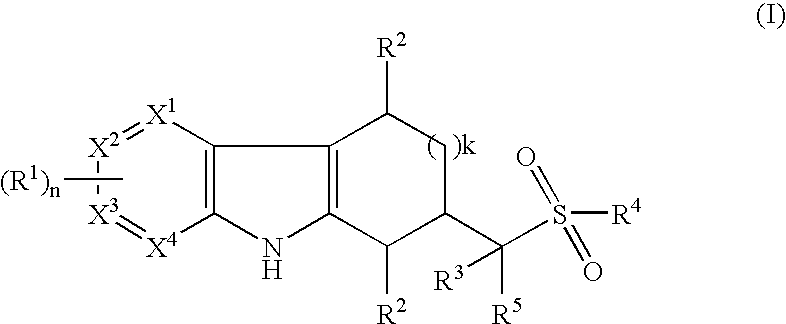
Tetrahydrocarbazoles and derivatives
a technology of tetrahydrocarbazoles and derivatives, applied in the field of compounds, can solve the problems of limiting the therapeutic potential of this approach, unphysiologically high levels of insulin, and ultimately reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
benzenesulfonyl-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-2-yl)-acetic acid methyl ester
[0307] To a stirred solution of 9 g (0.042 mol) of methyl phenylsulfonylacetate in 90 mL of MeOH at 0° C., 1.56 mL (0.008 mol, 0.2 eq) of a solution of sodium methoxide (5.4 M in MeOH) were added. After 15 min, 4.04 g (0.02 mol) of 2-cyclohexen-1-one were added. The reaction mixture was allowed to reach RT within 4 hours, diluted with saturated aqueous NH4Cl and extracted with EtOAc. The combined organic phases were dried over Na2SO4, filtered and evaporated. Column chromatography on silica gel with heptane / EtOAc 1:1 yielded 11.50 g (88%) of benzenesulfonyl-(3-oxo-cyclohexyl)-acetic acid methyl ester as a racemic mixture of diastereomers, light yellow oil, MS: 311 (MH+).
[0308] To 5 g (0.016 mol) of benzenesulfonyl-(3-oxo-cyclohexyl)-acetic acid methyl ester in glacial acetic acid (30 mL), 3.17 g (0.018 mol, 1.1 eq) of 4-chlorophenylhydrazine hydrochloride were added and the reaction mixture was ...
example 2
benzenesulfonyl-(6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-2-yl)-acetic acid methyl ester
[0309] In analogy to example 1.2, from benzenesulfonyl-(3-oxo-cyclohexyl)-acetic acid methyl ester and (4-fluoro-phenyl)-hydrazine hydrochloride was prepared benzenesulfonyl-(6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-2-yl)-acetic acid methyl ester as a racemic mixture of diastereomers, light yellow solid, MS: 402 (MH+).
example 3
(RS,SR)-2-benzenesulfonyl-2-(6-chloro-2,3,4,9-tetrahydro-1H-carbazol-2-yl)-propionic acid methyl ester
3.1
[0310] To a stirred solution of 2 g (6.44 mmol) of benzenesulfonyl-(3-oxo-cyclohexyl)-acetic acid methyl ester in 40 mL of DMF at 0° C., 283 mg (7.09 mmol, 1.1 eq) of NaH (60%) were added. The reaction mixture was stirred at this temperature for one hour, and 1.37 g (9.66 mmol, 1.5 eq) of methyliodide were added. After 2 hours at RT, the reaction mixture was poured into water and extracted with EtOAc. The combined organic phases were dried over Na2SO4, filtered and evaporated. The resulting two diastereomers were separated by column chromatography on silica gel with heptane / EtOAc 2:1, yielding 0.79 g (38%) of (RS,SR)-2-benzenesulfonyl-2-(3-oxo-cyclohexyl)-propionic acid methyl ester as a white solid, MS: 325 (MH+) and 0.71 g (34%) of (RR,SS)-2-benzenesulfonyl-2-(3-oxo-cyclohexyl)-propionic acid methyl ester as a white solid, MS: 325 (MH+).
3.2
[0311] To 0.14 g (0.43 mmol) (RS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com