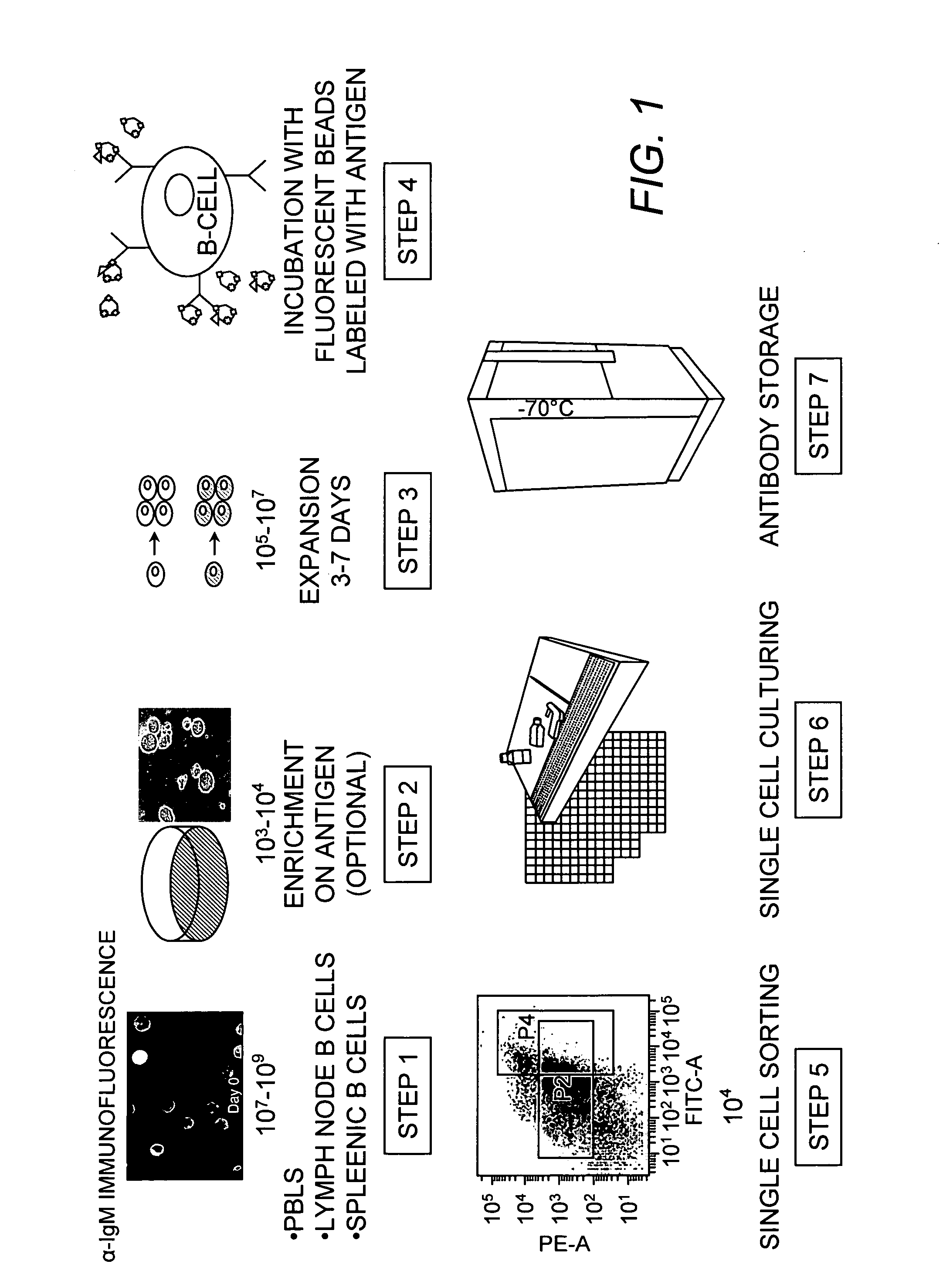
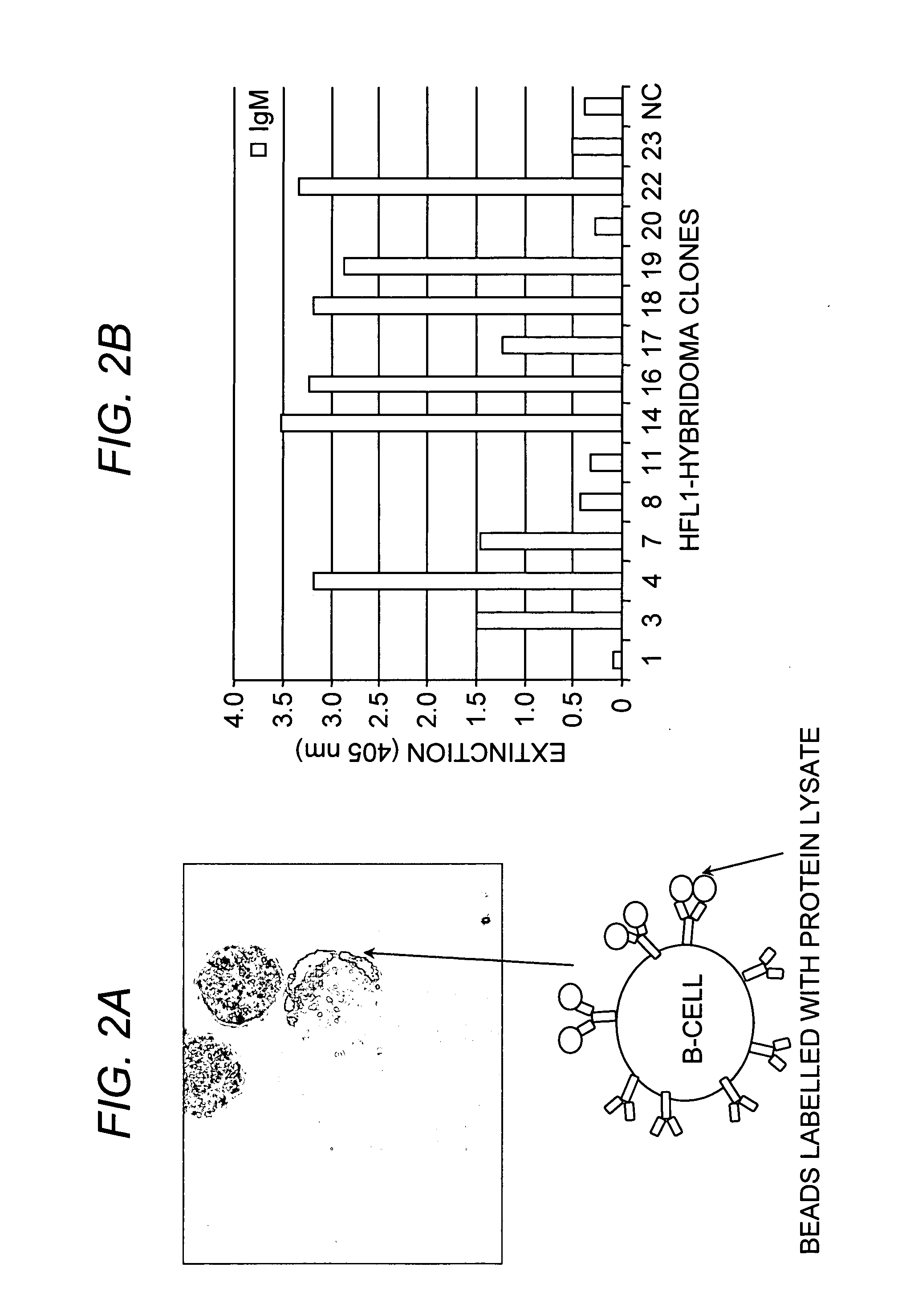
Method of producing a plurality of isolated antibodies to a plurality of cognate antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] A high-throughput, one-step selection method for producing a plurality of antigen-specific antibodies has now been developed. The method of the invention is advantageous over the art in that a plurality or collection of antibodies can be produced in one step and said antibodies are antigen-specific, species-specific, and have a high affinity to their cognate antigens. The method of the invention involves binding a plurality of antibody-producing B-cells from a mammal to a plurality of cognate antigens; isolating each bound antibody-producing B-cell and cognate antigen; amplifying nucleic acid sequences encoding each antibody, or fragment thereof, from the B-cells; introducing each nucleic acid sequence encoding each antibody, or fragment thereof, into an expression system for expressing an antibody to produce a plurality of isolated antibodies to a plurality of cognate antigens. While recombinant technology is desirable, conventional hybridoma technology can also be employed....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com