HAS-active ingredient conjugates
a technology of active ingredients and conjugates, which is applied in the field of conjugates of active ingredients, can solve the problems of preventing the medical use of these products, affecting the clinical use of many active ingredients of pharmaceuticals, and provoking life-threatening hypersensitivity reactions,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Selective Oxidation of the Reducing End Groups of the Hydroxyethyl Starch
[0141] For the selective oxidation of the reducing end groups of the hydroxyethyl starch (130 kD and 10 kD), the same were dissolved in a minimal quantity of water and reacted with different quantities of an iodine solution and a KOH solution.
[0142] The mixture was stirred until the colour indicating I2 disappeared. This procedure was repeated several times in order to achieve the addition of a larger quantity of the iodine solution and KOH solution. The solution was subsequently purified using an Amberlite IR 120 Na+ cation-exchanger resin, dialysed for 20 hours against distilled water (dialysis tube with an exclusion limit of 4-6 kD) and lyopholized.
[0143] The degree of oxidation was determined in each case using the process disclosed in Somogyi, N. (Method in Carbohydride Chemistry, Vol. 1, (1962) p. 384-386, hereby incorporated by reference). The protocols of the oxidation reaction are reproduced in Tabl...
example 2
Binding of HES with Oxidized Reducing End Groups to HSA in the Aqueous Phase
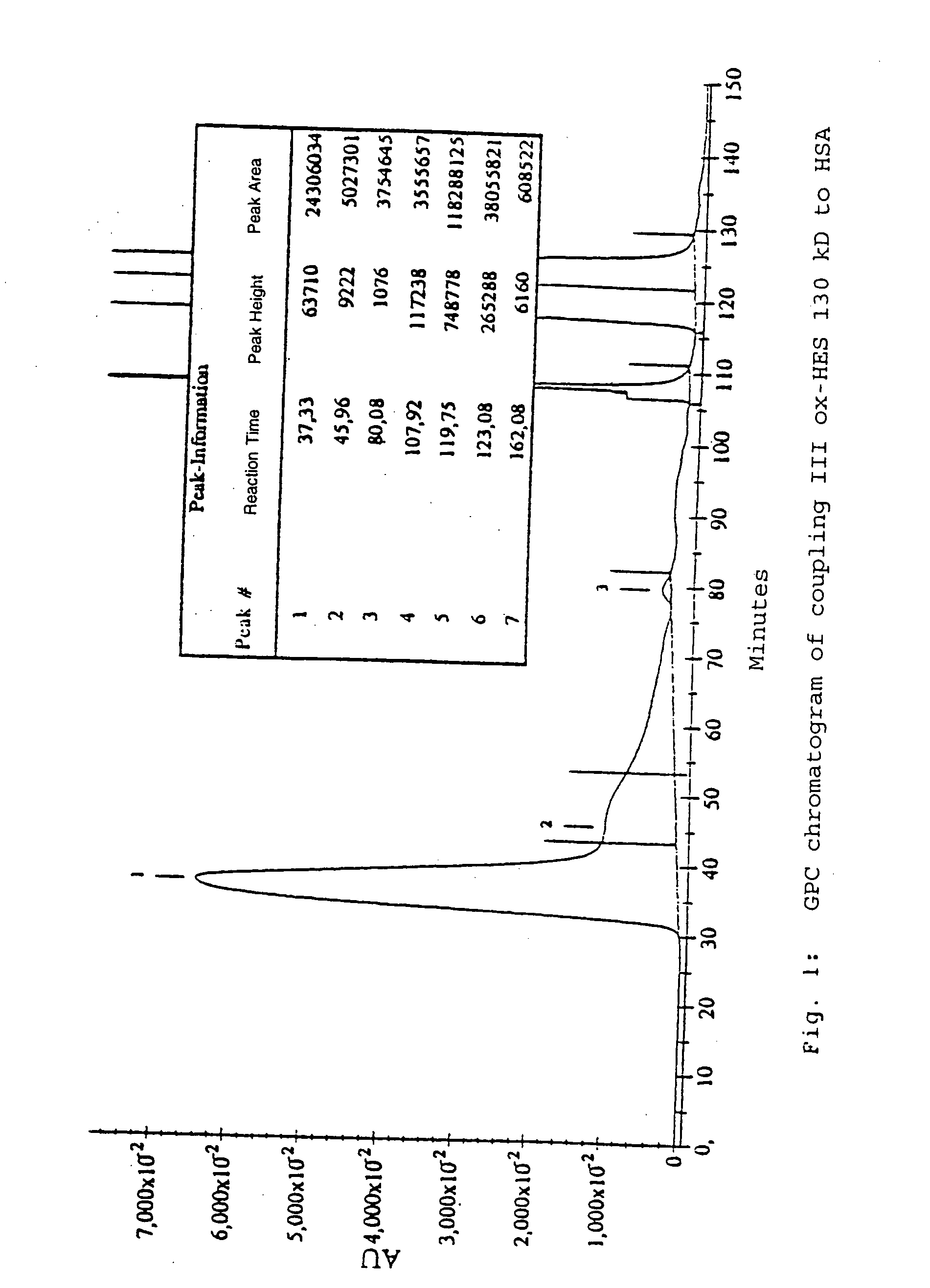
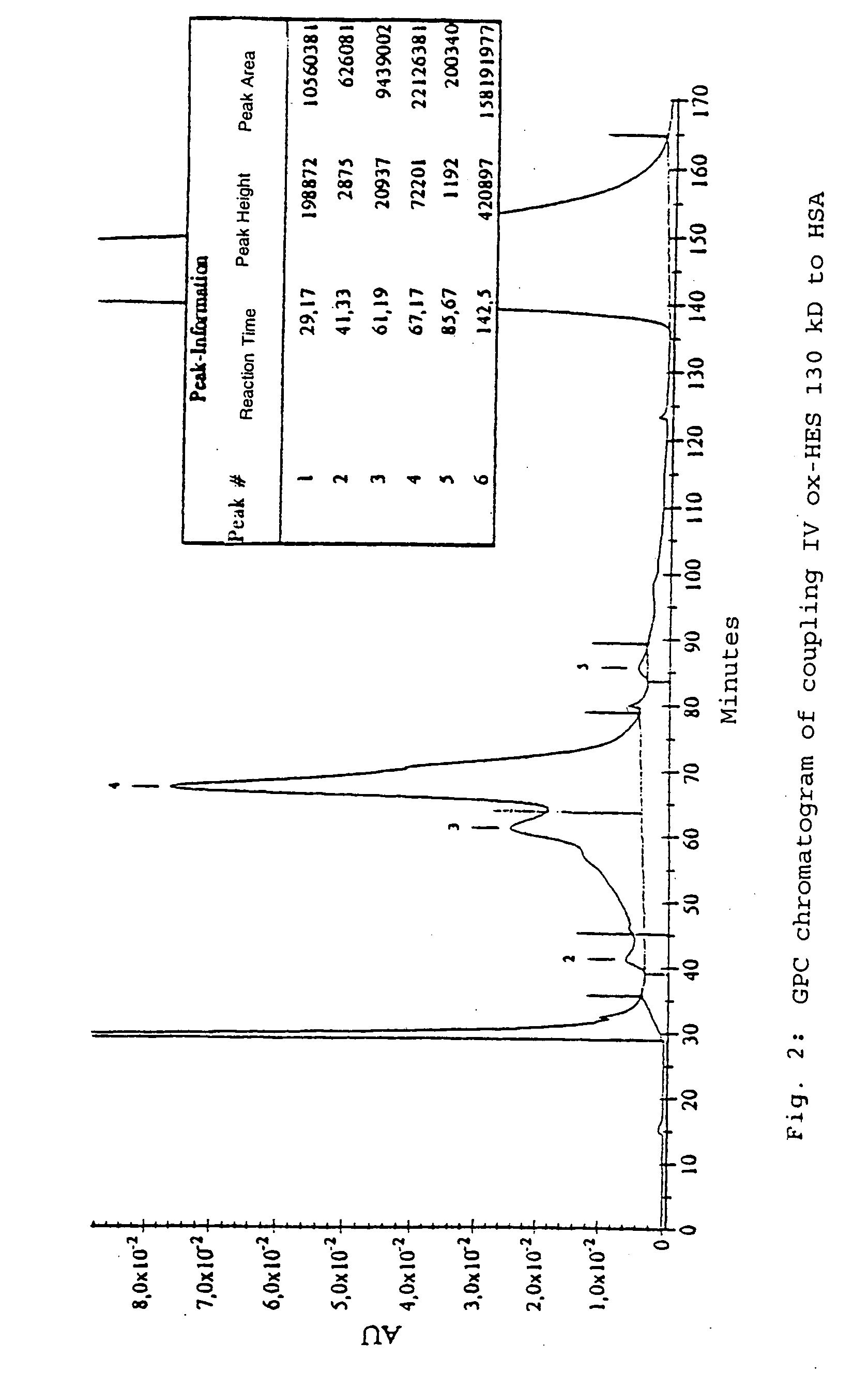
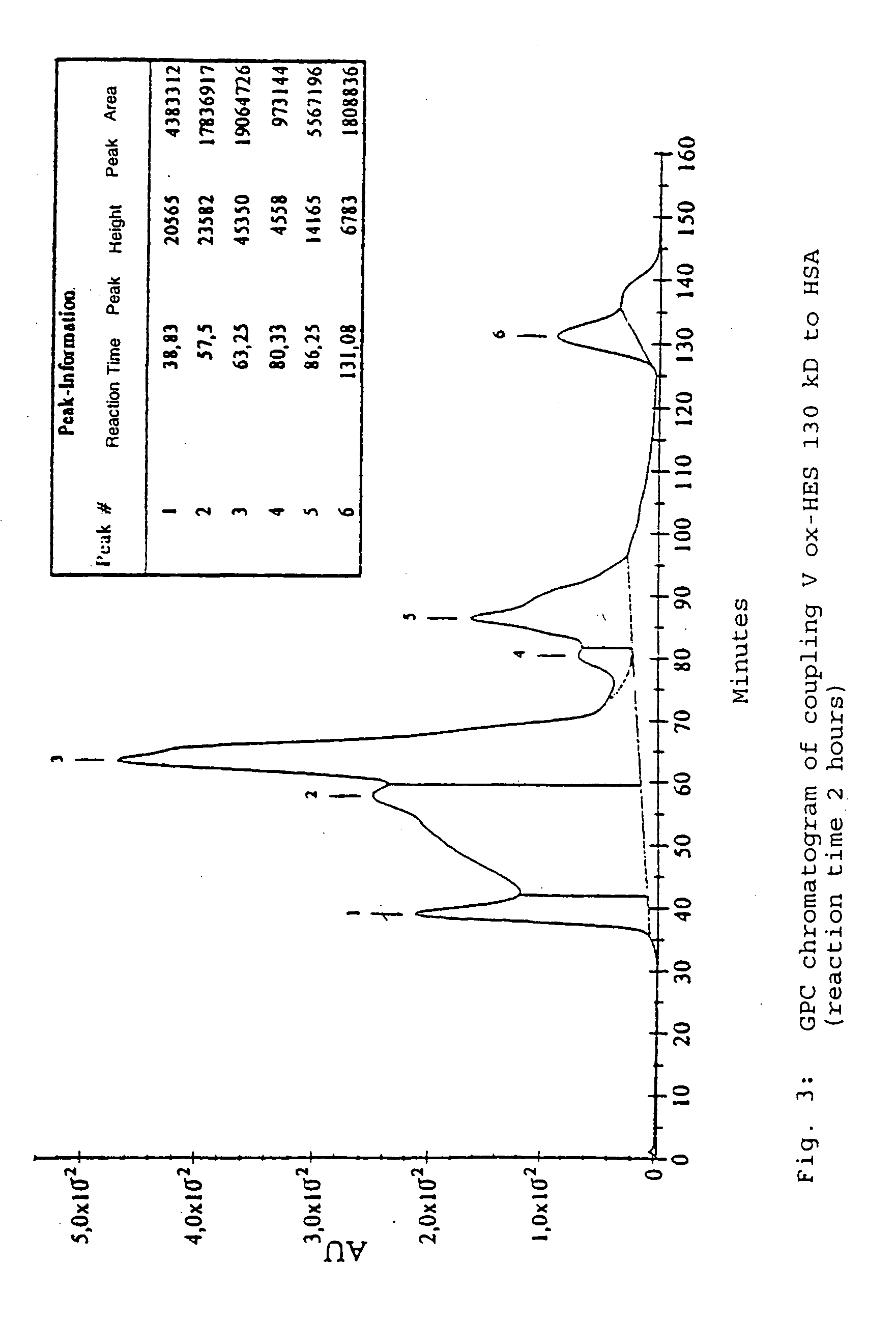
[0147] For the coupling, hydroxyethyl starch with oxidized reducing end groups (ox-HES) and HSA were completely dissolved in water. When the solution was clear, EDC dissolved in water was added. After activation by EDC accompanied by moderate stirring, further quantities of EDC were added. Where appropriate, the reaction was activated with HOBt and left to stand overnight. The product was purified for 15 hours by dialysis against distilled water and subsequently lyophylized (called Process A in the following).
[0148] The protocols of the coupling reaction are in Table 2.
TABLE 2Coupling reactions between HES (130 kD and 10 kD) with oxidized reducing endgroups and HSA under different conditions (Process A; number in brackets in theHES column reproduces the oxidation process according to Table 1).ox-HESActi-Process AHSA(Mn)EDCHOBtSolventvationReactionCoupling I300mg100mg (1)25mg100mgH2O / dioxane1.5 hours4 hou...
example 3
Direct Binding of HES to HSA in the Aqueous Phase
[0150] The principle of this reaction is based on the formation of Schiff s bases between HES and amino groups of the protein, the reaction being controlled through the reaction of the Schiff s bases to the corresponding amine by NaBH4 (called Process B in the following).
[0151] For this, HES was dissolved completely in a small amount of water. To this end, HSA dissolved in borate buffer, pH 9.0, was added. NaBH4 was added to this solution and the whole left at room temperature accompanied by stirring. A further aliquot of HES 130 kD, followed by further NaBH4, was added. After the reaction was finished, dialysis and freeze-drying was carried out as described.
[0152] The protocols of the individual tests are summarized in Table 3.
TABLE 3Direct coupling between HES (130 kD and 10 kD) and HSA under differentconditions (Process B).Process BHSAHES (Mn)NaBH4Buffer pHReaction timeCOUPLING I50mg500mg500mgNa2HPO4, 0 ml48 hoursHES 1307.5 × ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| average molecular weight | aaaaa | aaaaa |
| average molecular weight | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com