Medical device package kit
a medical device and package technology, applied in the field of medical device packages, can solve the problems that conventional medical device packages do not meet all or even most of the requirements described above in a cost effective manner, and achieve the effect of preventing repeated use and cost effectiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
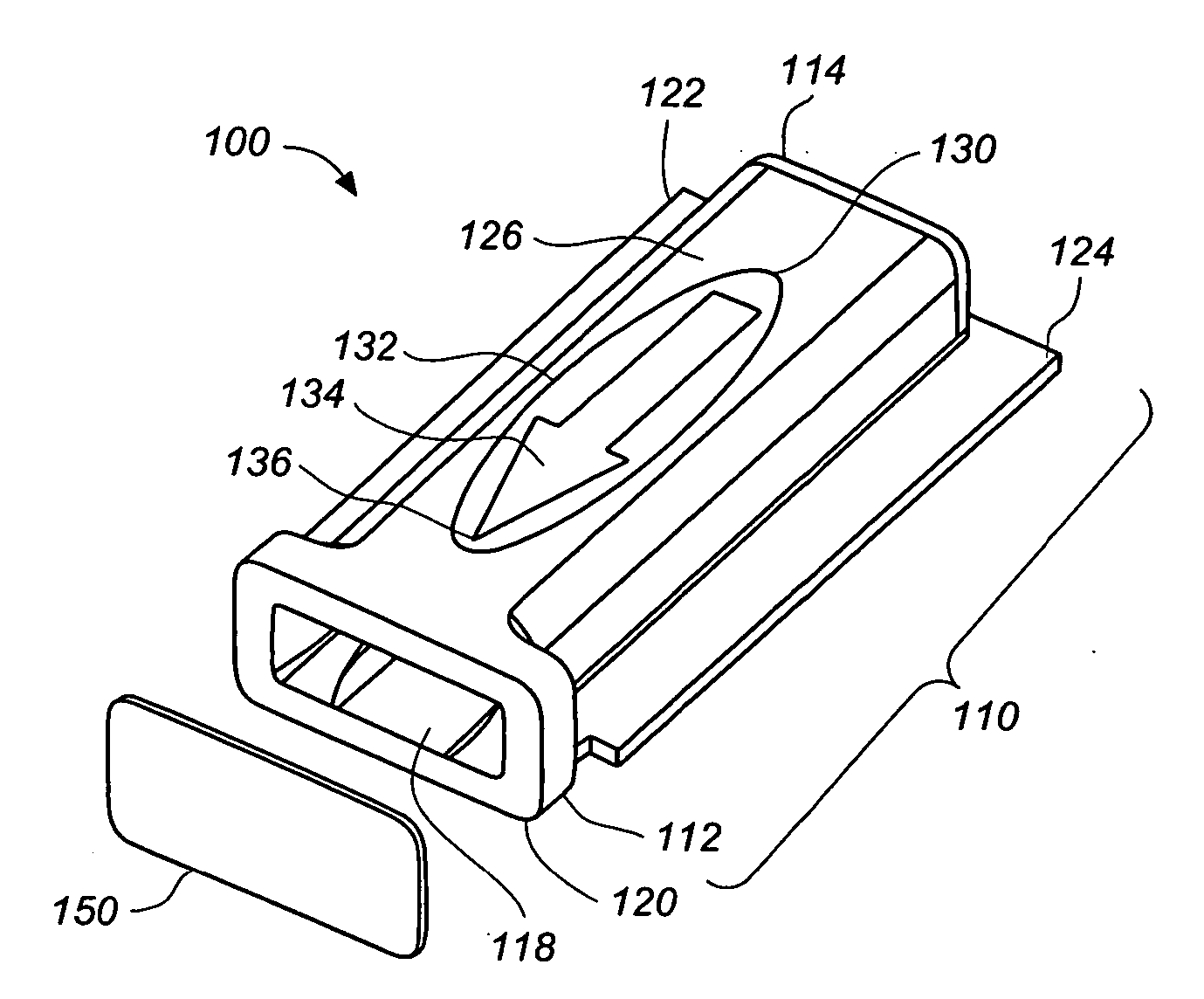
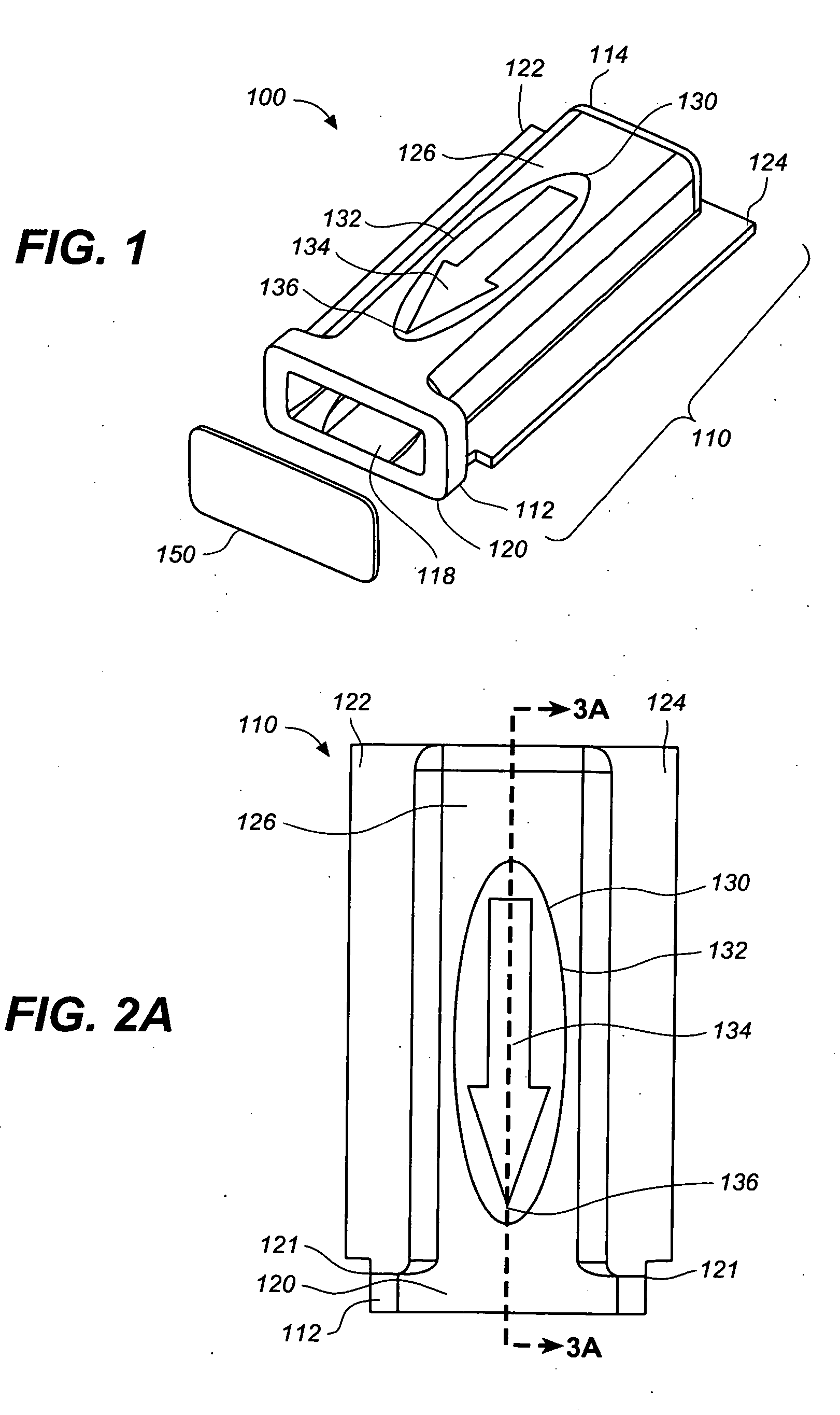
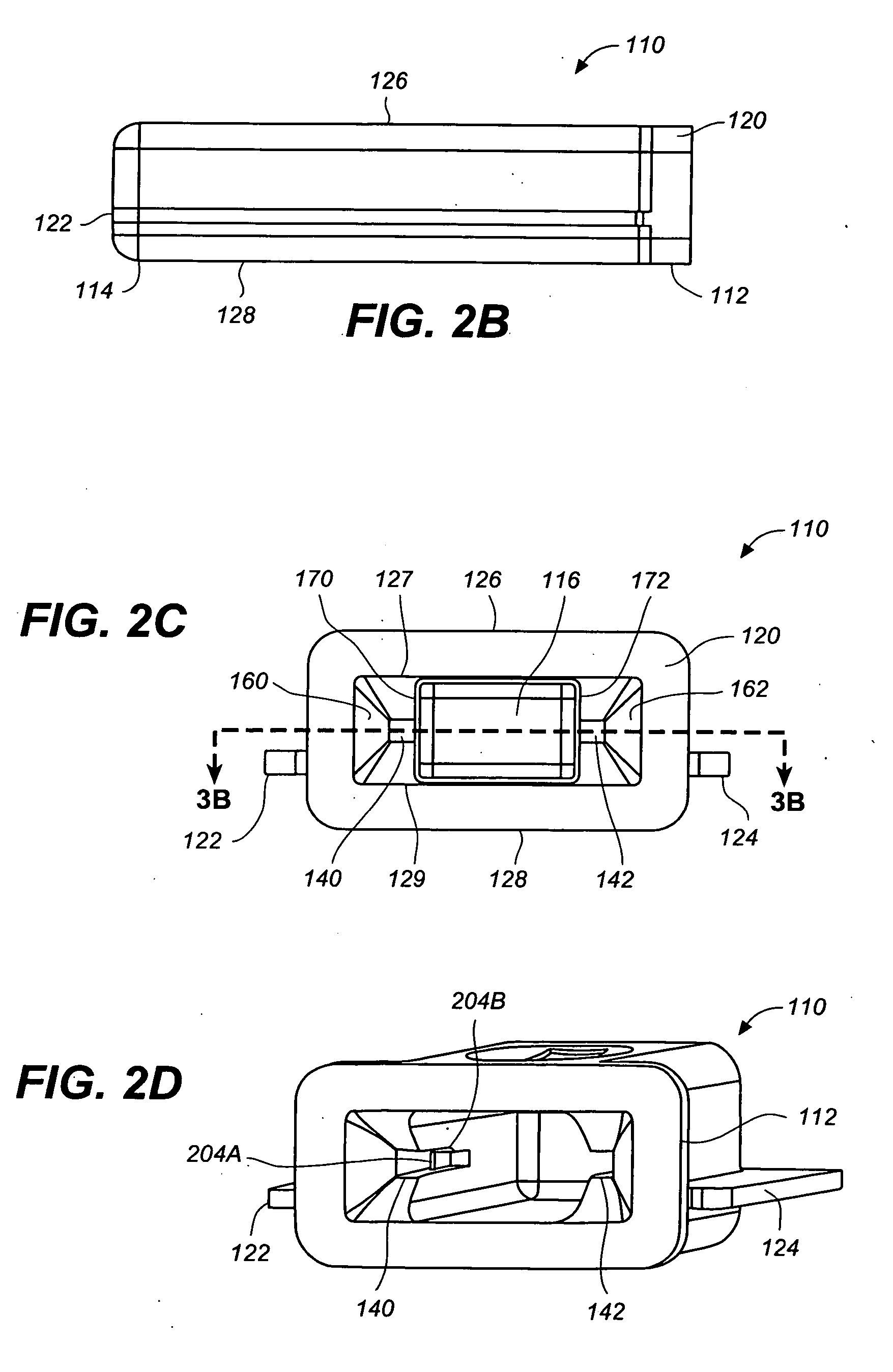
[0047] FIGS. 1, 2A-2D, 3A and 3B are various simplified views of a medical device package 100 according to an exemplary embodiment of the present invention. Medical device package 100 includes a main cap member 110 and a minor cap member 150.
[0048] Main cap member 110 includes a cavity 116 therein, a proximal end 112 and a distal end 114. Cavity 116 has a cavity opening 118 at the proximal end 112 of the main cap member 110 and is configured to receive, and to securely and removably retain, a medical device (e.g., integrated medical device 300, illustrated in FIGS. 4A and 4B), at least partially therein.
[0049]FIGS. 4A and 4B are simplified perspective and side views, respectively, of an exemplary integrated medical device 300 that can be securely and removably contained within medical device package 100. Integrated medical device 300 includes a test strip 304 and a dermal tissue penetration member 302, as illustrated in FIGS. 4A-4B. Test strip 304 has a reaction area (not shown) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com