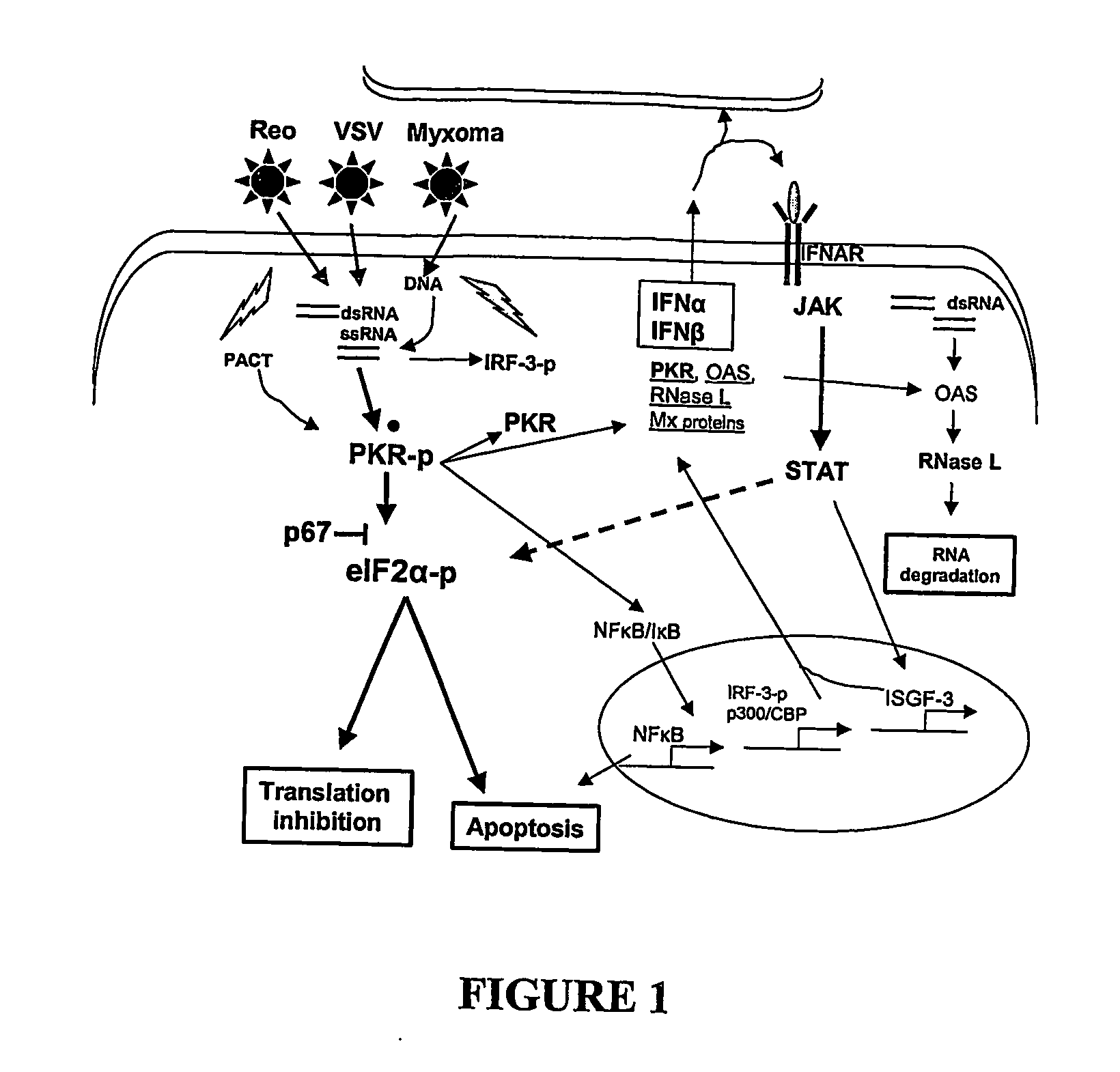
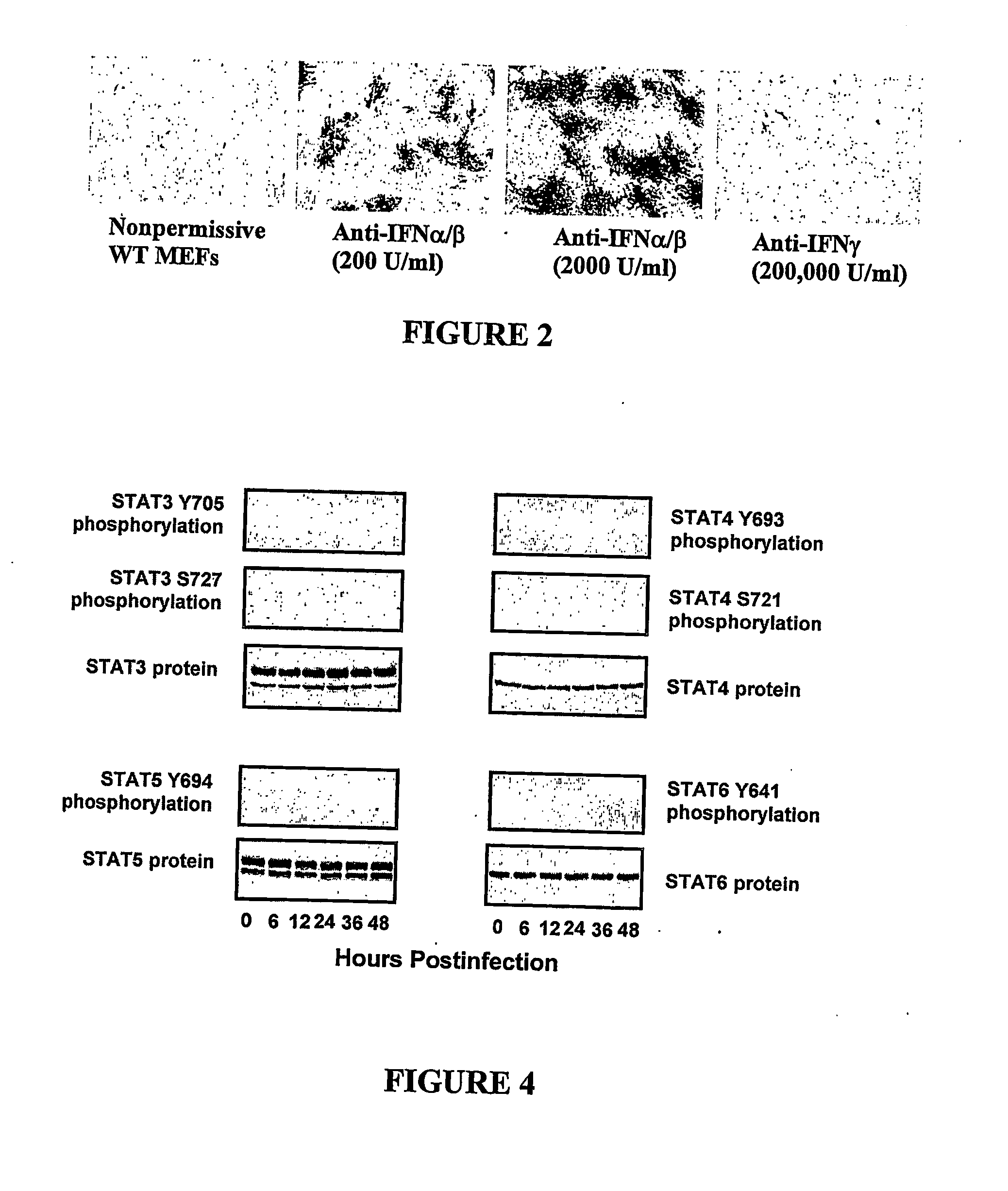
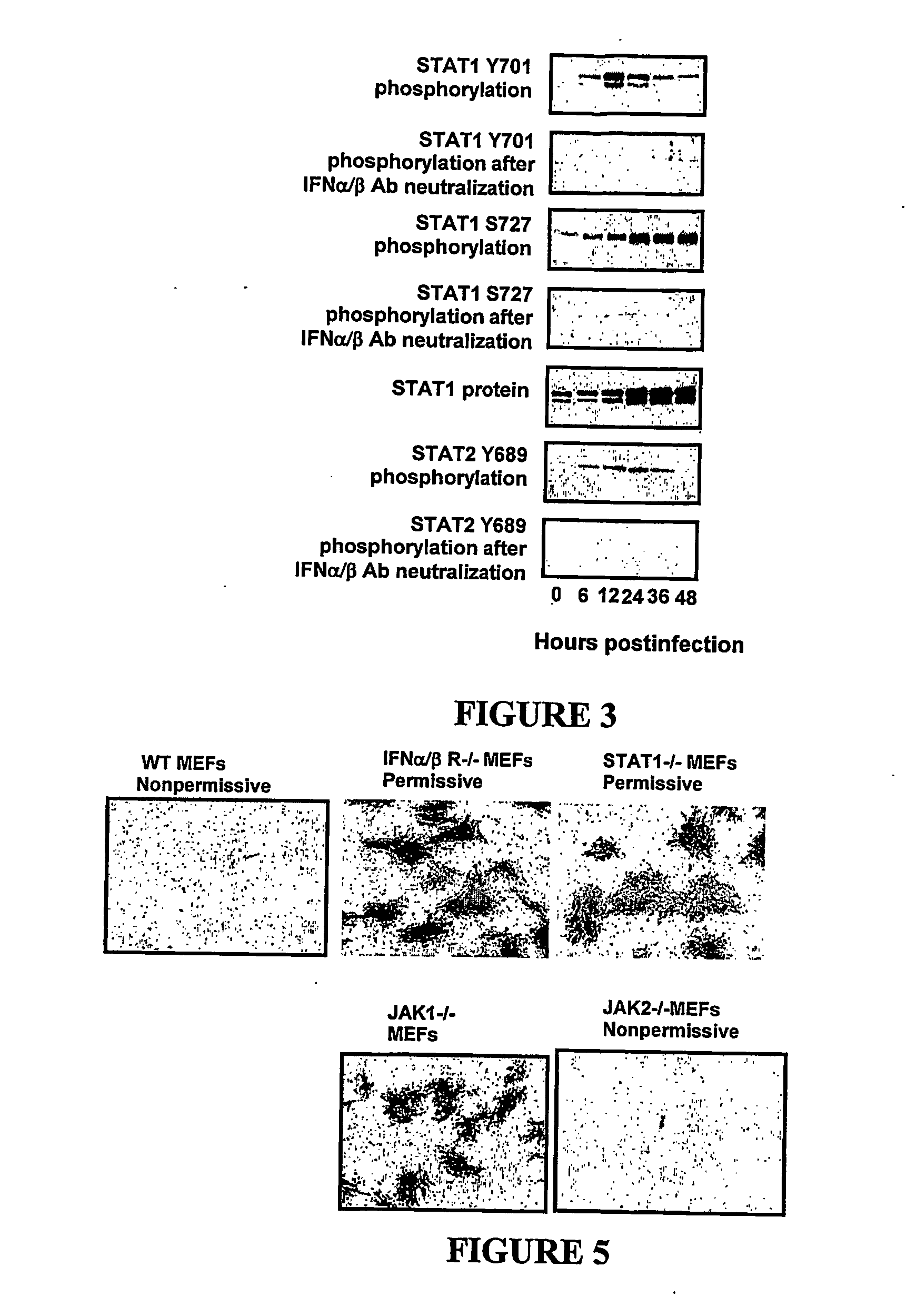
Use of myxoma virus for the therapeutic treatment of cancer and chronic viral infection
a technology of myxoma virus and cancer, applied in the field of myxoma virus therapy, can solve the problems of inability unable to achieve effective cancer treatment, and unable to meet the needs of patients with cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Virus Strains
[0116] Viral strains used include wildtype MV, MV modified to express either green fluorescence protein (“GFP”) or β-galactosidase (“LacZ”), and killed (“dead”) MV. Viruses were prepped and titred using standard techniques.
Cell Strains
[0117] Mouse experiments were performed using mouse embryo fibroblasts (“MEFs”) derived from a wild-type mouse, and from the following mouse knockouts: IFNα / β receptor homozygous knockout; STAT1 homozygous knockout; PKR heterozygous; RNaseL heterozygous knockout; Mx1 heterozygous knockout; triple PKR / RNaseL / Mx1 homozygous knockout.
[0118] Human experiments were performed on BGMK control cells and human tumour cell lines HT29, HOP92, OVCAR4, OVCAR5, SK-MEL3, SK-MEL28, M14, SKOV3, PC3, DUI45, CAKI-1, 786-0, T47D, MDAMB 435, SF04, U87, A172, U373, Daoy and D384 as described in Stojdl et al., Cancer Cell (2003) 4: 263-275.
Methods
[0119] Generally, assays and experiments were performed as described in Lalani et al. Virology (1999) 256: 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase contrast micrograph | aaaaa | aaaaa |
| fluorescent image | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com