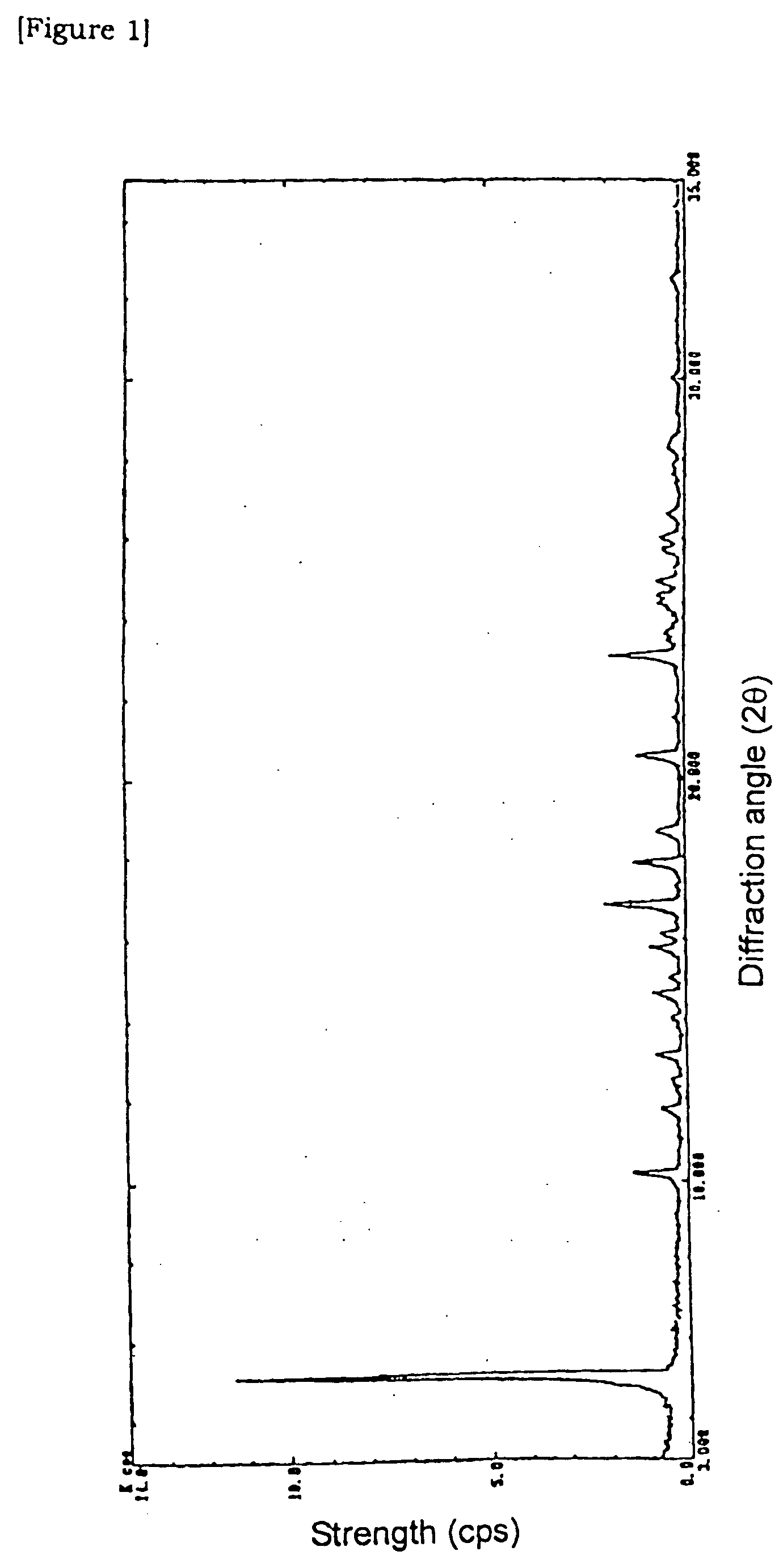
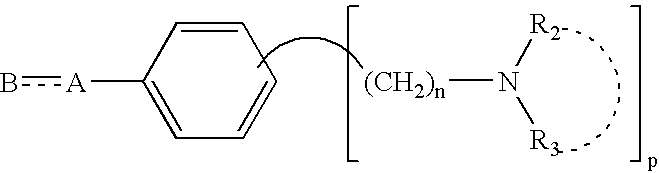
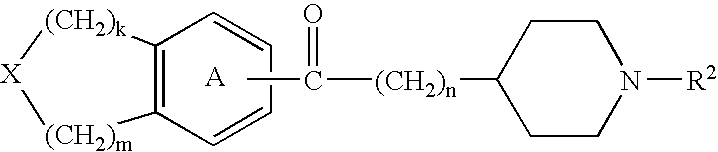Agents and crystals for improving excretory potency of urinary bladder
a technology of urinary bladder and agents, which is applied in the field of drugs, can solve the problems of insufficient clinical efficacy of distiginine, inability to completely satisfy the needs of pregnant women, and inability to use bethanechol, etc., and achieves the effects of improving the excretion of the urinary bladder, high efficacy, and high efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference examples 1-30
[0481] According to per se known methods, compounds of Reference Examples 1-30 were obtained as depicted in the following Table, wherein R′═H.
TABLE 1Reference Example No.ArRnY1H22H23H24H25H26H27H28H29H210H2
[0482]
TABLE 2Reference Example No.ArRnY11H212H213H214H215H216H217H218H219H220H2
[0483]
TABLE 3Reference Example No.ArRnY21H222H223H224H225H226H227H228H2
[0484]
TABLE 4ReferenceExampleNo.2930
reference example 15-1
8-[3-[1-[(3-fluorophenyl)methyl]-4-piperidinyl]-1-oxopropyl]-1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]quinolin-4-one (Compound of Reference Example 15)
[0485]
[0486] 1) To thionyl chloride (300 mL) was added 3-(1-acetyl-4-piperidinyl)propionic acid (88.2 g, 0.443 mol) in small portions under ice cooling. The mixture was stirred at room temperature for 10 minutes, and then thionyl chloride was distilled off at 25° C. under reduced pressure. Diethyl ether was added to the residue and then evaporated in vacuo to give a yellow solid. Again, diethyl ether was added, and the solid was crushed with a spatula, and ether was evaporated in vacuo to give 3-(1-acetyl-4-piperidinyl)propionic acid chloride as crude light yellow powder. This light yellow powder and 1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]quinolin-4-one (64.0 g, 0.369 mol) were suspended into 1,2-dichloroethane (200 mL), into which aluminum chloride (162 g, 1.21 mol) was added in small portions at room temperature. The mixture was stir...
reference example 15-2
8-[3-[1-(phenyl methyl)-4-piperidinyl]-1-oxopropyl]-1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]quinolin-4-one (Compound of Reference Example 17)
[0499]
[0500] 8-[3-[(4-Piperidinyl)-1-oxopropyl]-1,2,5,6-tetrahydro-4H-pyrrolo[3,2,1-ij]quinolin-4-one [obtained in section 2) of Reference Example 15-1 and benzyl bromide were treated in the same manner as in section 3) of Reference Example 15-1 to give colorless powder, which was crystallized from ether-isopropyl ether to give the title compound as colorless crystals having mp. 103-104° C.
[0501]1H-NMR (CDCl3) δ 1.20-1.75 (8H, m), 1.85-2.05 (2H, m), 2.71 (2H, t, J=7.6 Hz), 2.80-2.95 (3H, m), 3.02 (2H, t, J=7.6 Hz), 3.22 (2H, t, J=8.6 Hz), 3.49 (2H, s), 4.13 (2H, t, J=8.6 Hz), 7.20-7.35 (5H, m), 7.67 (1H, s), 7.71 (1H, s).
[0502] Elemental analysis for C26H30N2O2:
[0503] Calcd: C, 77.58; H, 7.51; N. 6.96
[0504] Found: C, 77.30; H, 7.49; N, 7.20
[0505] The above-mentioned title compound was dissolved in ethanol, to which was added 1.5 equivalent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com