Methods and probe combinations for detecting melanoma
a technology of melanoma and probe combinations, applied in the field of methods and probe combinations for detecting melanoma, can solve the problems of significant morbidity, no consensus can be reached, and diagnostic ambiguity has significant adverse effects on patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Probe Selection
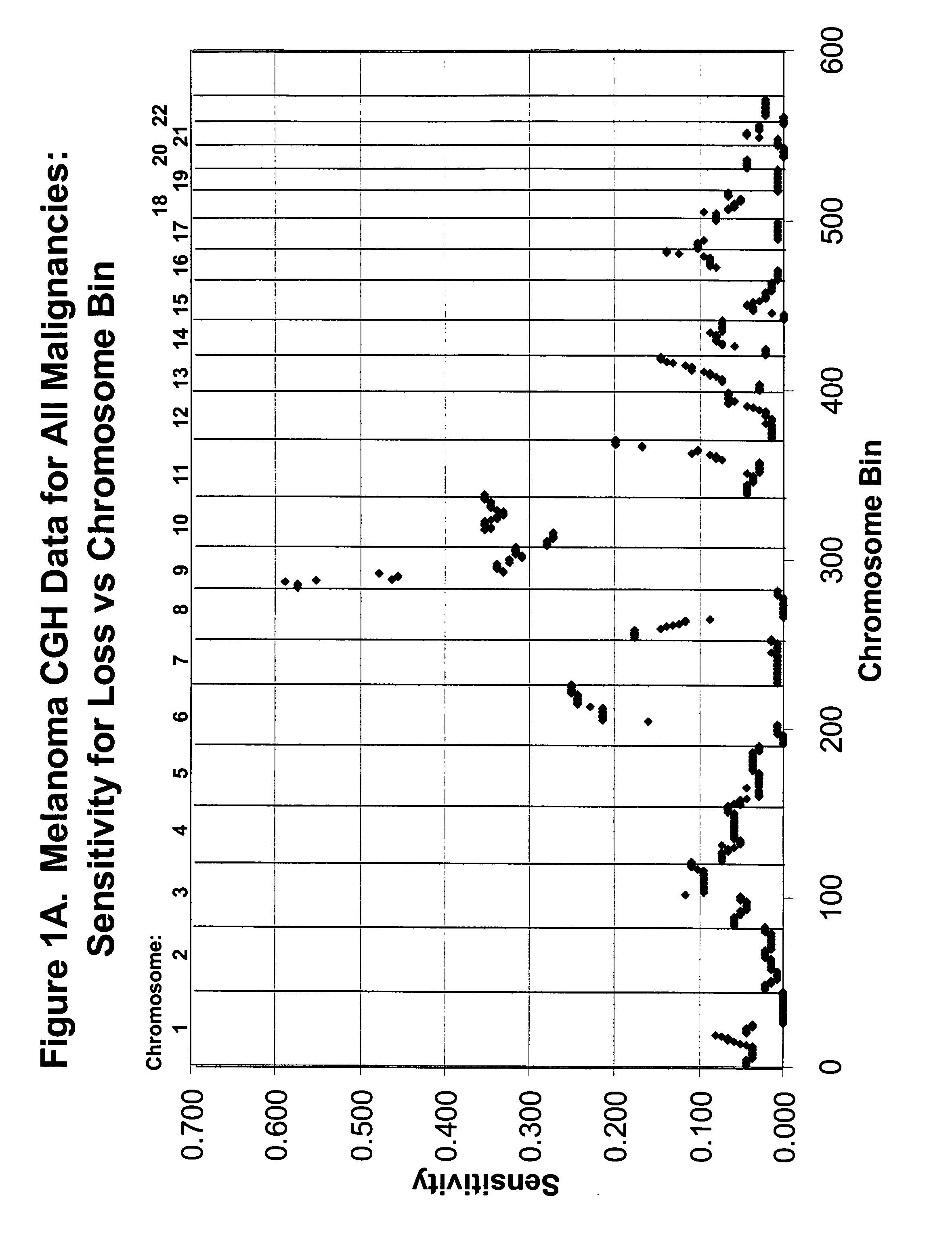
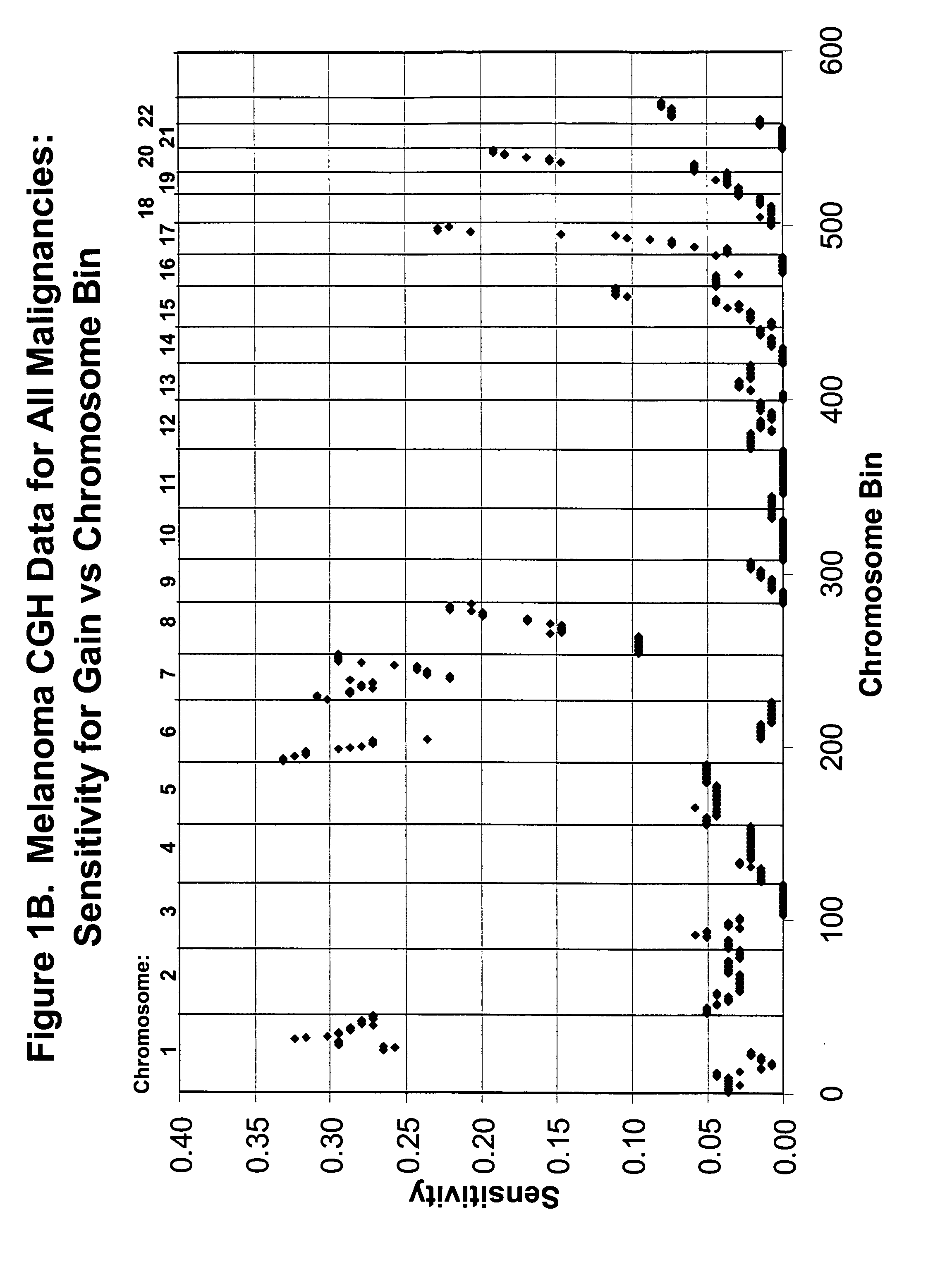
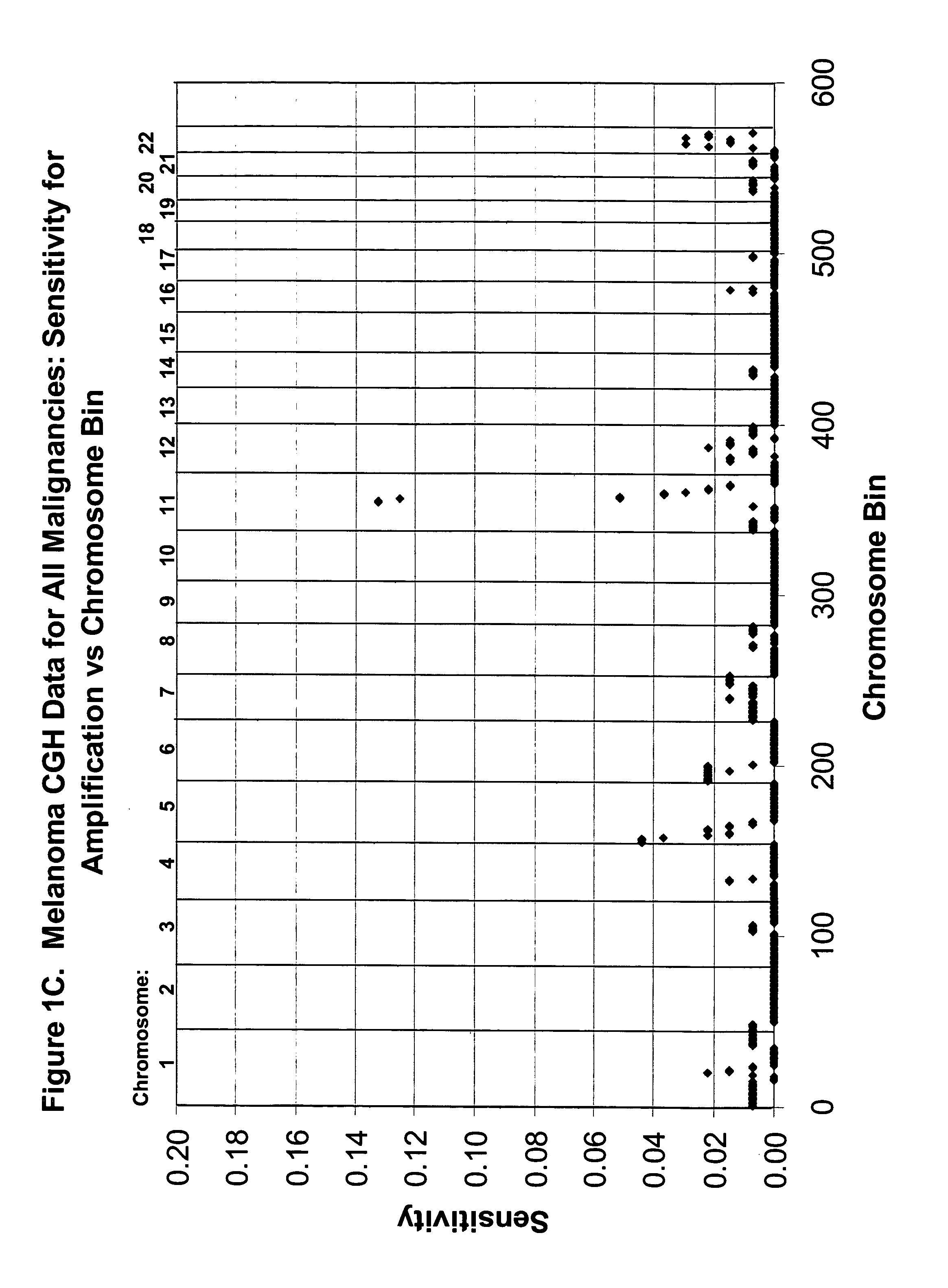
[0097] CGH Database. The CGH data on which probe selection was based had been acquired at the University of California, San Francisco and has been published previously (Bastian et al, Am J Pathol. 163:1765-70, 2003). The data were from 136 primary cutaneous melanoma specimens (63 superficial spreading melanomas (SSM), 30 lentigo maligna melanomas, (LMM), 23 acral-lentiginous (ALM), 4 nodular melanomas (NM), 10 not classifiable (NC), and 6 melanomas arising within a nevus) and 53 benign nevi specimens (19 blue nevi, 7 congenital nevi, 27 Spitz nevi). The genome from the lp telomere to the 22q telomere (chromosomes X and Y omitted) was divided into 571 ‘bins’ according to the Giemsa banding pattern of chromosomes and the CGH ratio corresponding to each bin was interpreted as reflecting chromosomal gain (tumor to reference fluorescence intensity ratio) (ratio>1.2), and loss (ratio<0.8). These thresholds were based on hybridizations of normal DNA versus normal DNA as pu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com