Humanized antibodies against cd3
a technology of humanized antibodies and cd3 is applied in the field of humanized antibodies against cd3 to achieve the effect of limiting the clinical utility of this therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
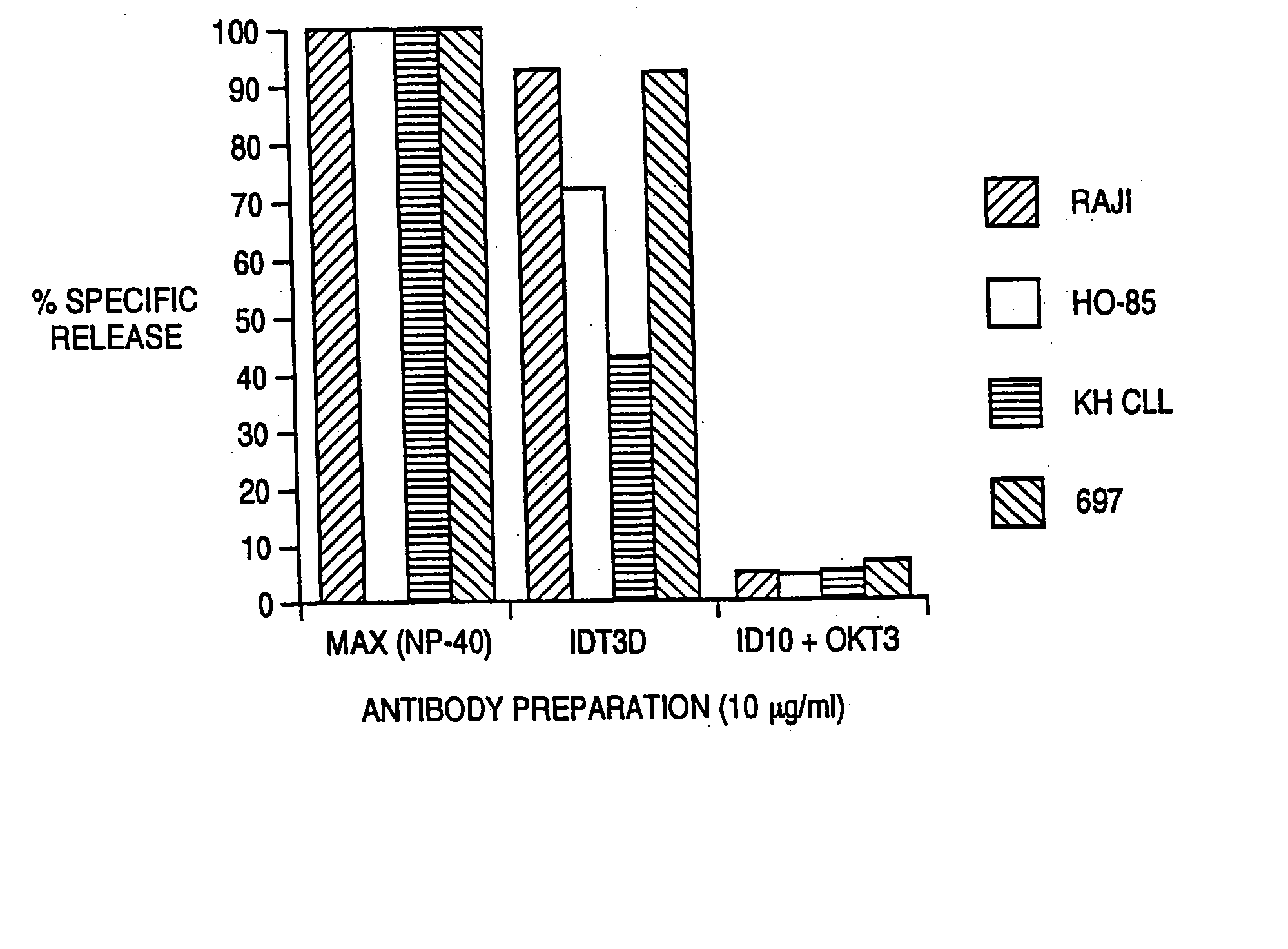
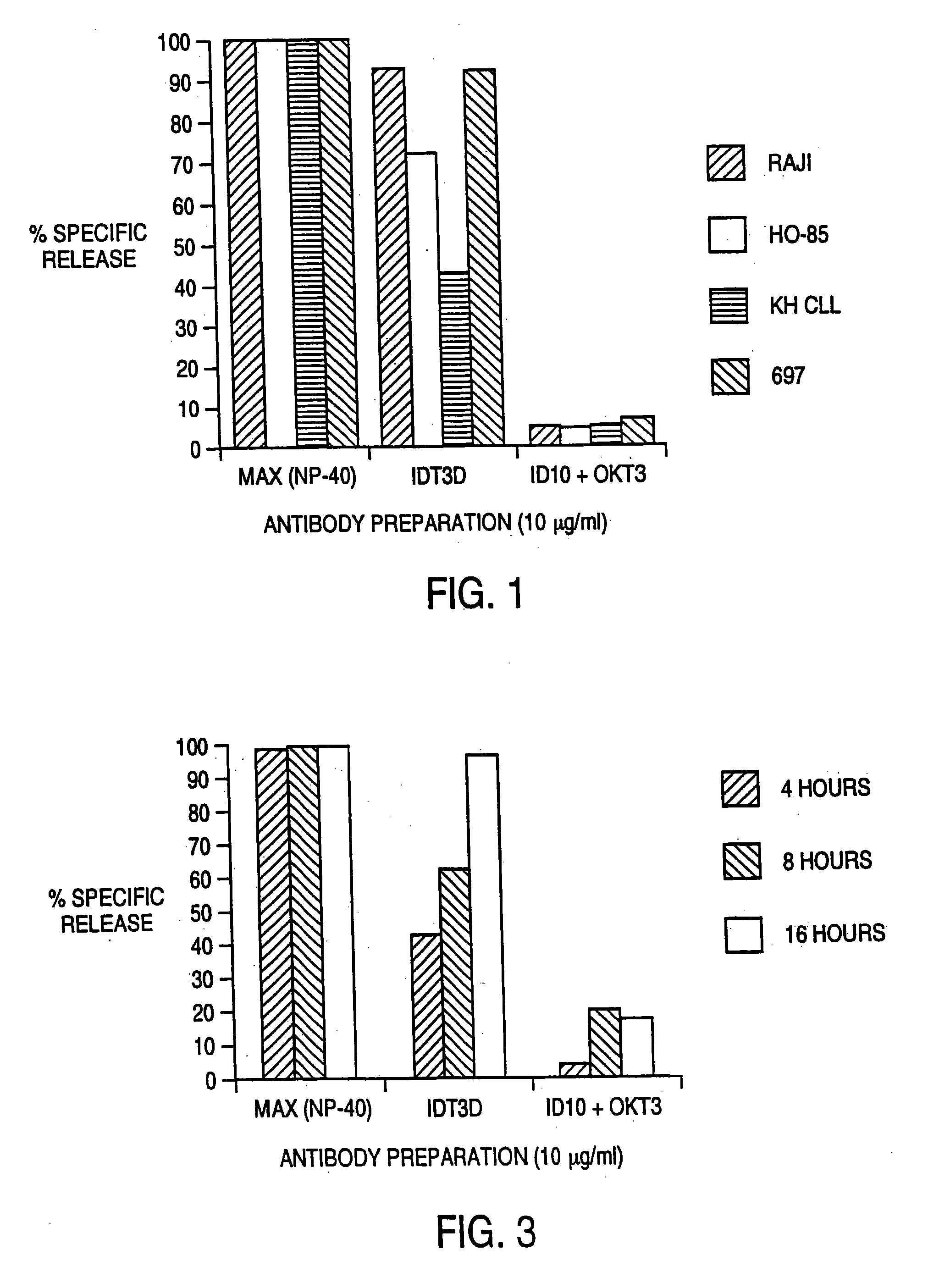
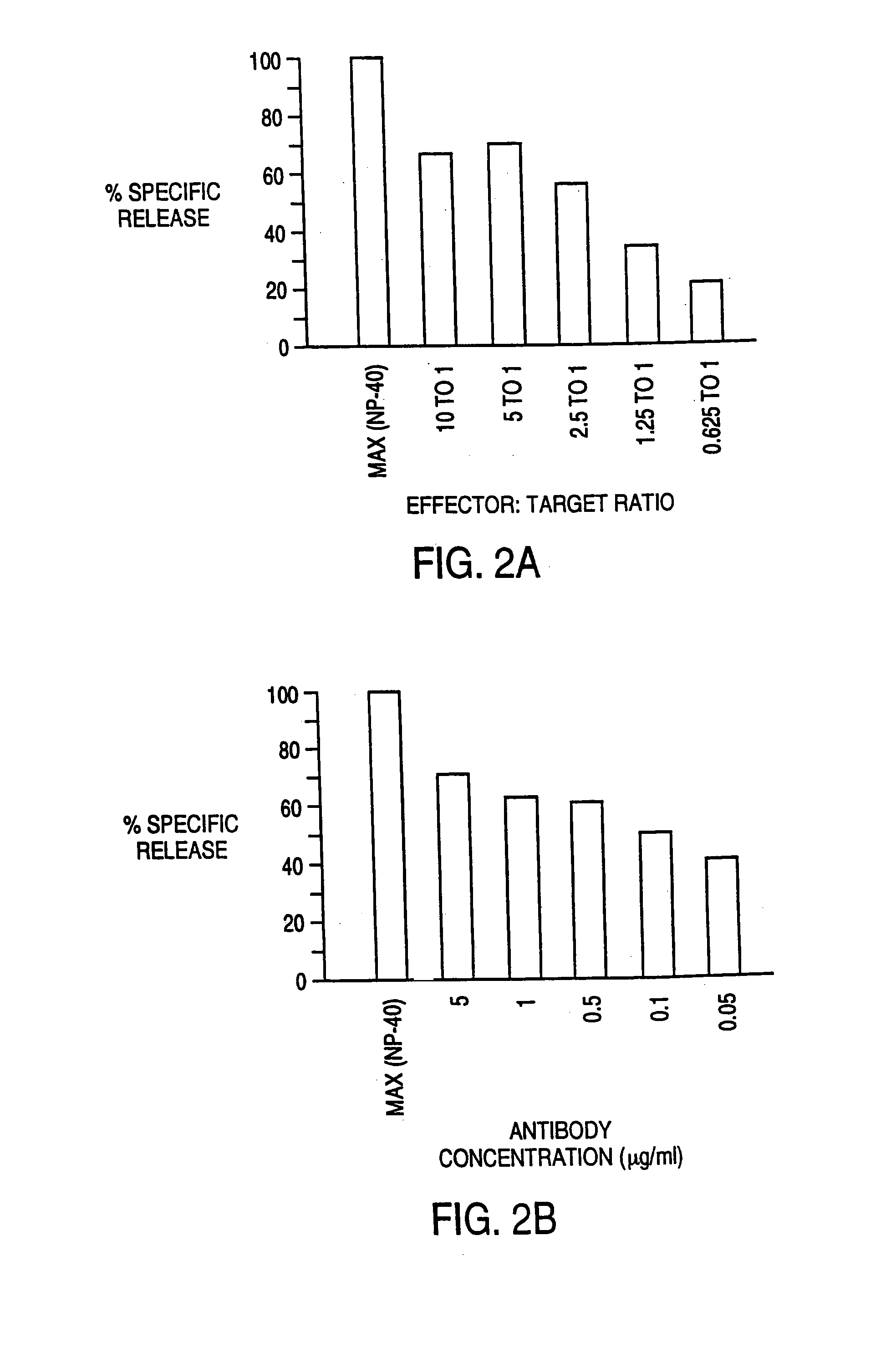
[0096] The ability of IDT3-D to induce the elimination of malignant B cells by T cells was evaluated in vitro. The assay used was a 51 chromium-release cytotoxicity assay. Target malignant B cells (10.sup.7 cells in 1 ml) were labeled during a 1 hour incubation with 100 μCi 51Cr. T cells from normal donors were incubated in vitro with IL-2 or IL-2 and anti CD3 antibody for 3-7 days before use as effector cells. T cells were added to 51Cr-labeled malignant B cells along with antibody. This mixture was incubated for 4 hours, and cell free supernatant was removed and evaluated for the presence of released 51Cr Cr by gamma counting. Maximum release was determined by evaluating supernatant obtained from wells that had been treated with detergent (NP-40) that induces the lysis of all cells. Background release was determined by evaluating 51Cr levels from samples that had target malignant B cells and T cells but no antibody. Specific release of 51Cr indicated lysis of the 51Cr-containing t...
example 2
In Vivo Efficacy of 1DT3-D Bispecific Antibody
[0103] This example describes an in vivo trial of the bispecific antibody 1DT3-D. Normal donor human peripheral blood lymphocytes were activated in vitro in the presence of OKT3 (2 μg / ml), and recombinant IL-2 (300 μg / ml). CB-17 scid / scid mice (Itoh et al., Cancer 72, 2686-2694 (1993)) were injected subcutaneously with 5×106 Raji cells mixed with 5×106 activated lymphocytes. 24-hr later, mice were injected with bispecific antibody, a monospecific antibody component of the bispecific antibody or no antibody. Mice were examined daily for the development of tumors of at least 0.5 cm at the site of tumor injection. Mice remaining tumor-free after 60 days were scored as negative and mice developing tumors within 60 days as positive. Control untreated mice always developed tumors within 21-28 days.
[0104] In a first experiment, 5 mice were treated with 10 μg / mouse of bispecific antibody 24 hours after inoculation with the mixture of malignant...
example 3
Generation of a Monoclonal Antibody Against the Human CD3 Antigen
[0113] The 1DT3-D antibody described in Example 1 incorporated OKT3 as the binding moiety having an affinity for effector cells. The present example describes the isolation of an alternative antibody, M291, for use as the effector-cell binding component in a bispecific antibody.
[0114] Human peripheral blood mononuclear cells (PBMC) were activated with PHA and IL-2 to expand T cells. Activated T cells were used as immunogens in Balb / C mice. Hybridomas were generated from the spleens of these mice by standard methods. These hybridomas were screened for antibodies that could stimulate PBMC to proliferate in vitro. Anti-CD3 antibodies with the appropriate Fc cause T cells in PBMC to proliferate. One of these hybridomas, M291, was isolated and found to secrete an antibody of the isotype IgG2a / kappa that could activate T cells to proliferate. The purified antibody M291 competes with another anti-human CD3 antibody, OKT3, (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com