Pharmaceutical formulations containing lipoic acid derivatives
a technology of lipoic acid and derivatives, which is applied in the direction of anhydride/acid/halide active ingredients, biocide, drug compositions, etc., can solve the problem of no specific guidance regarding the selection of suitable pharmaceutically acceptable carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
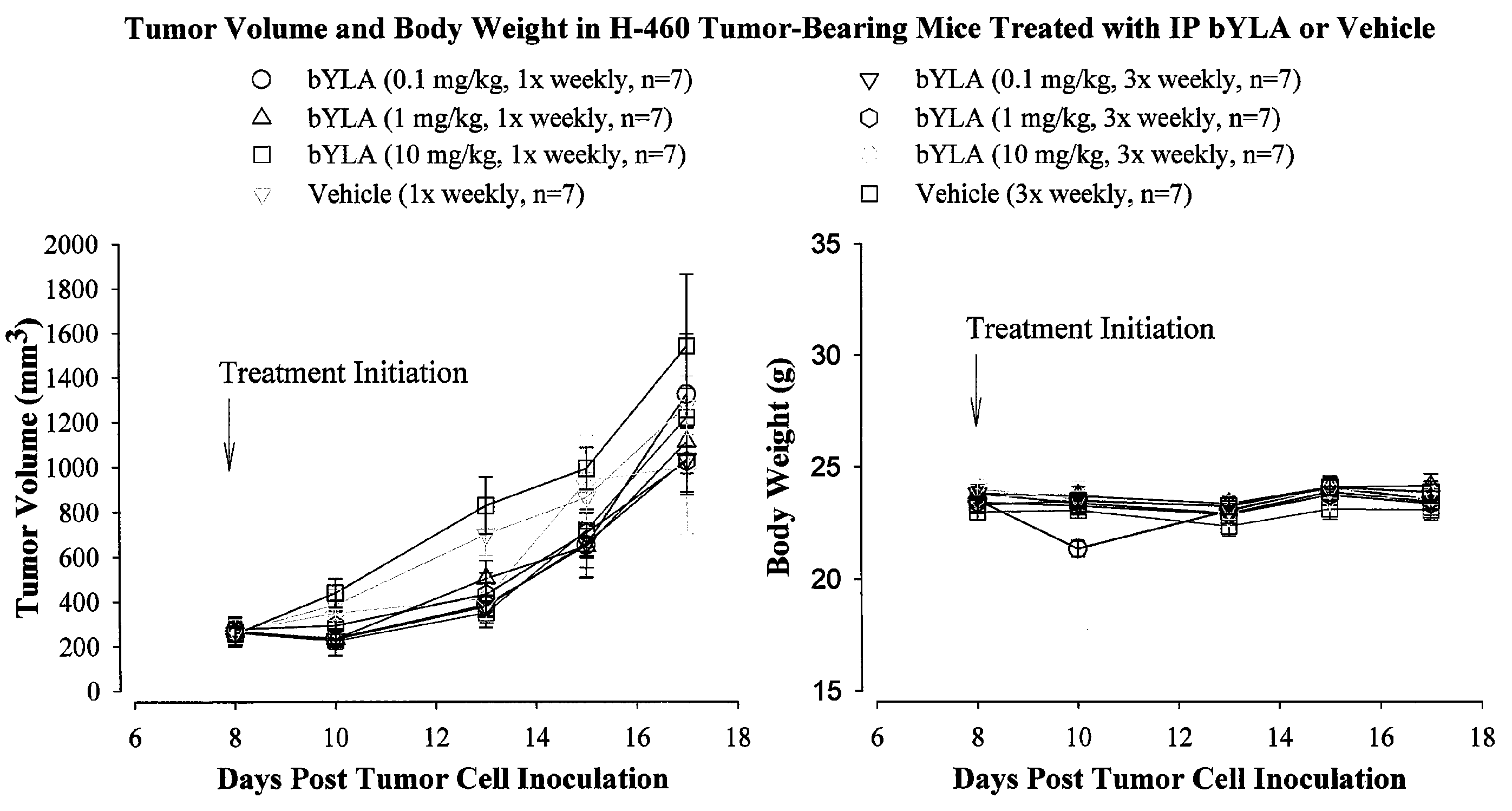
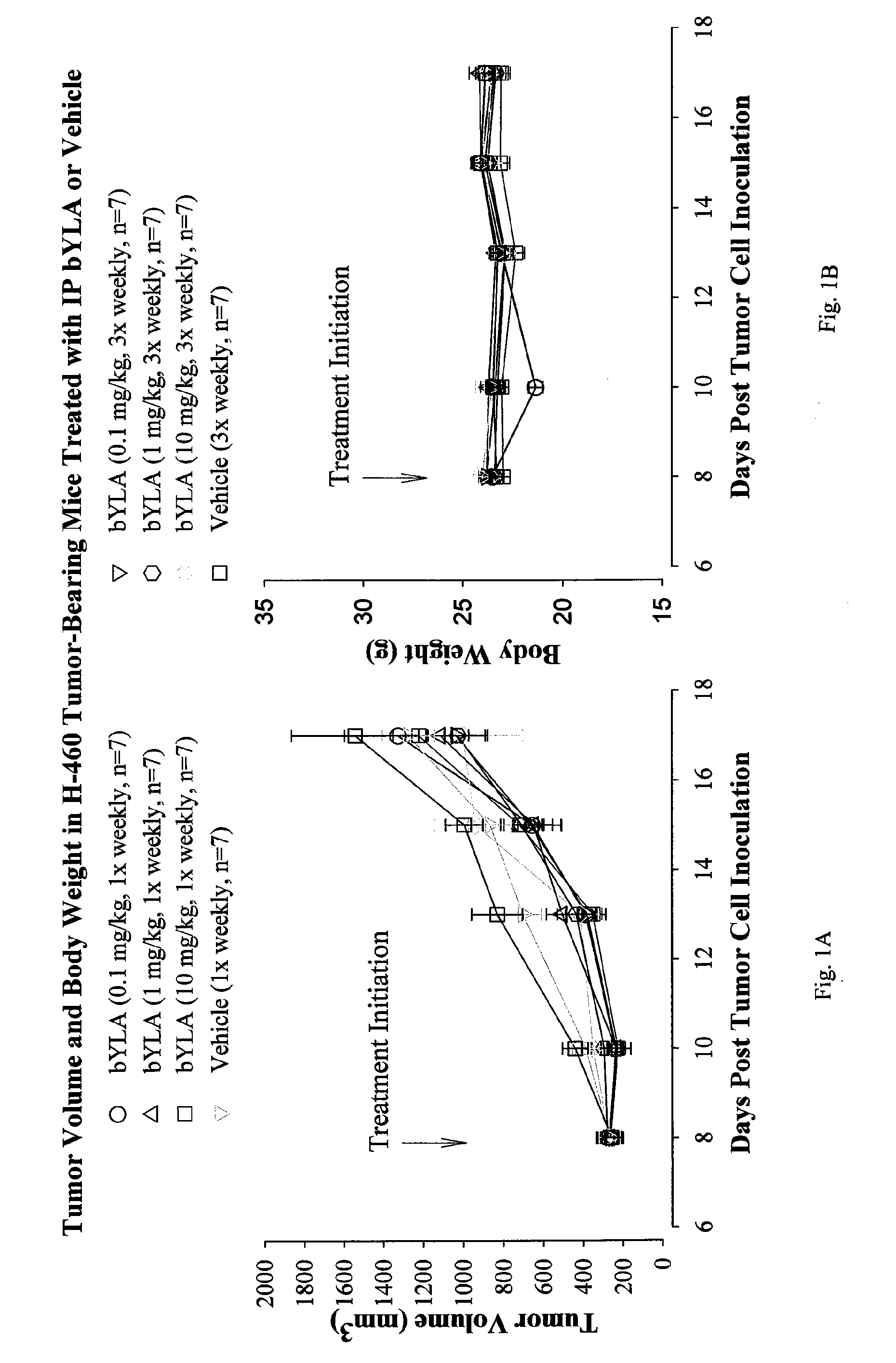
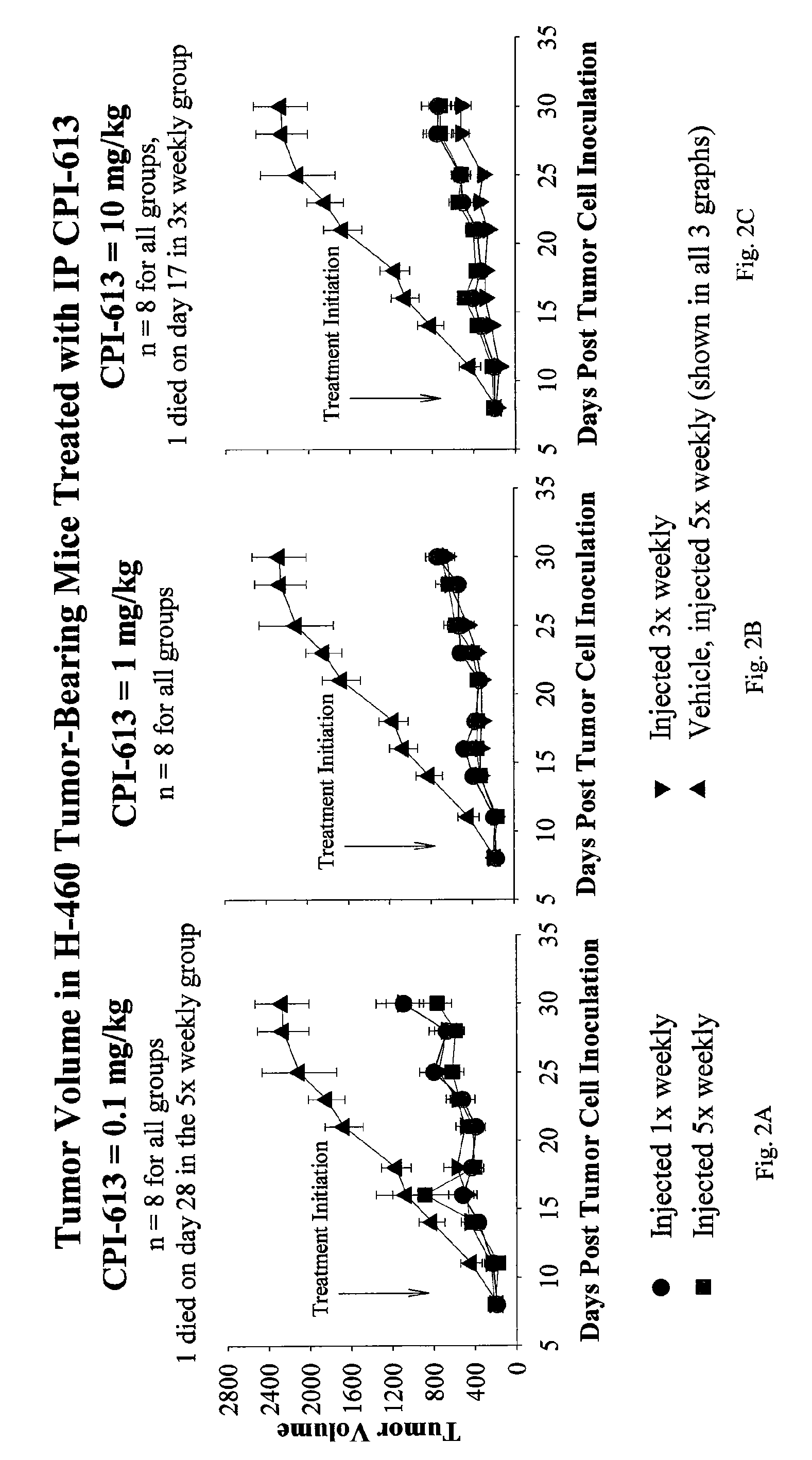
[0055]Bis-benzyl lipoate was provided in a concentrated form at a concentration of 50 mg / mL dissolved in 1M triethanolamine (TEA). The stability of the drug product was assessed by visual observation and by high-performance liquid chromatography (HPLC) assessment, performed at the beginning and the end of the study. The physical appearance did not change and the purity was found to be >99% pure, both at the beginning and the end of the study. The concentrated bis-benzyl lipoate solution was diluted to an appropriate concentration with 5% dextrose (D5W) to formulate 0.1, 1 and 10 mg / kg doses of bis-benzyl lipoate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com