Differential Immunoassay
a technology of immunoassay and immunoassay, applied in the field of differential immunoassay, can solve the problems of difficult to diagnose a heart attack within six hours of the onset of chest pain with a single test, difficult to perform single test, and high value of single test, so as to increase the sensitivity of assay and reduce the value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Two-Conjugate, Qualitative Homogeneous Assay for Heart Attack
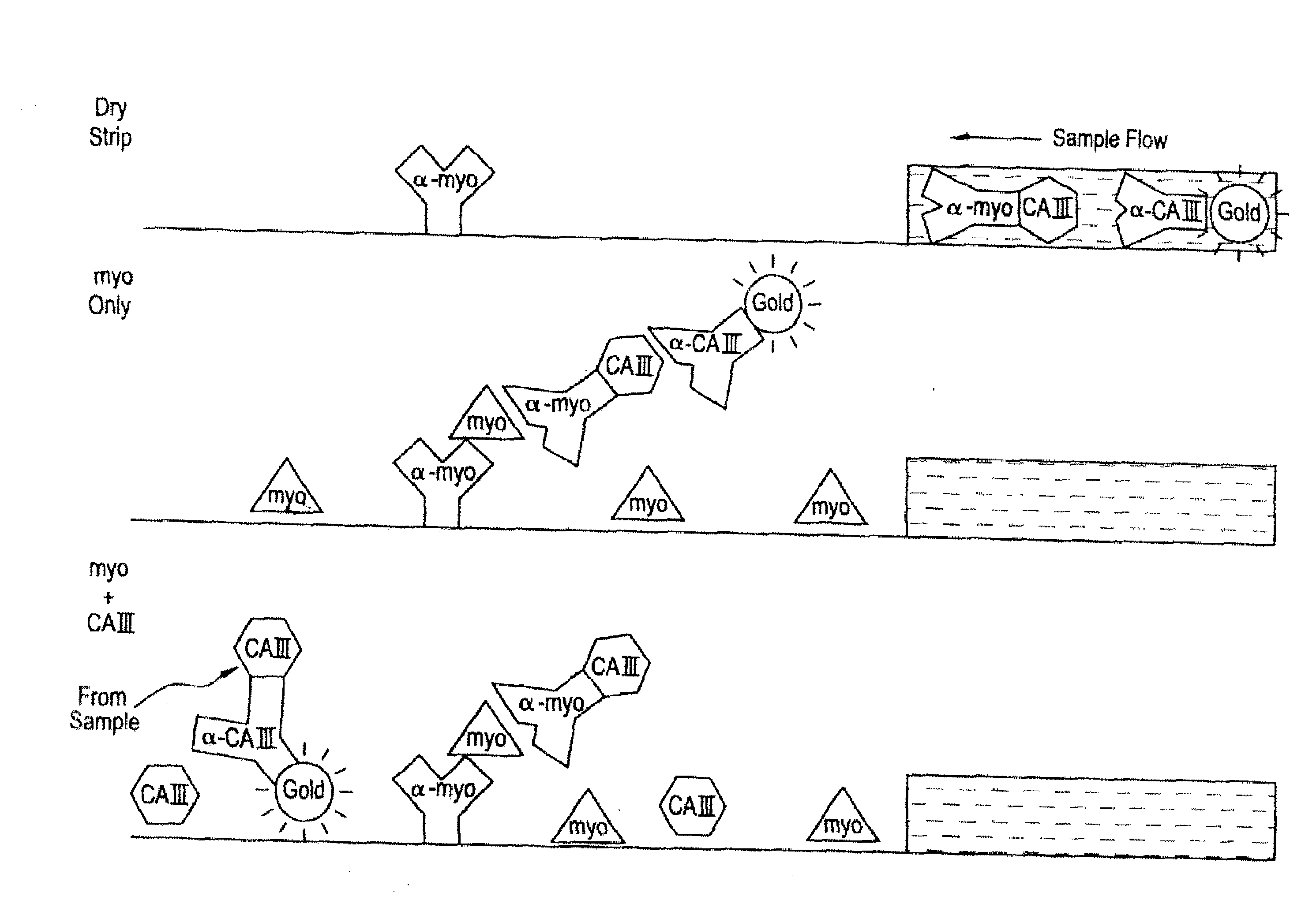
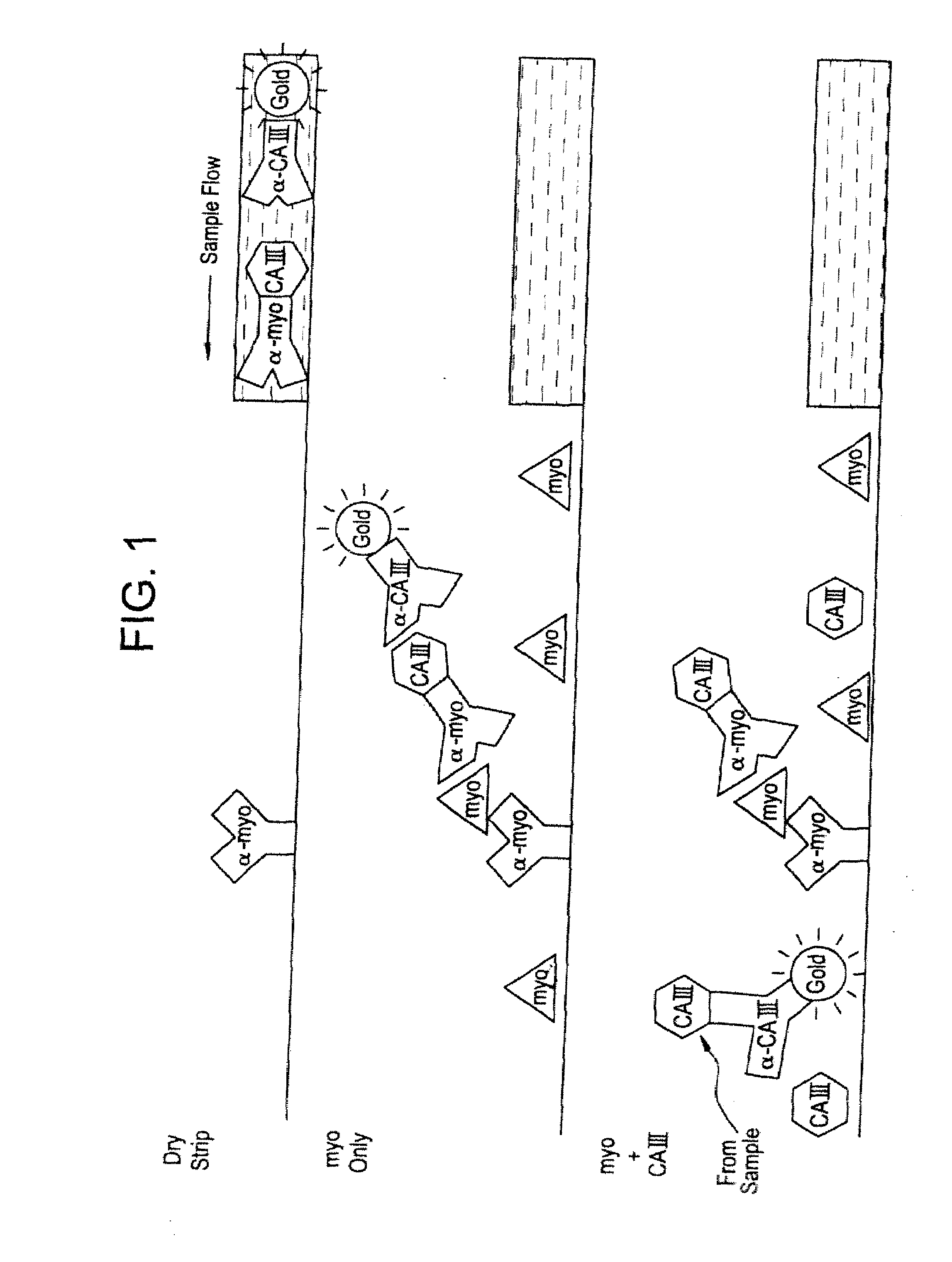
[0190]FIG. 1 illustrates the design and operation of a membrane strip-format assay which is positive for myoglobin only if carbonic anhydrase III is absent. Present in the detection zone of the test strip is analyte labeling reagent in the form of two mobile reagents: 1) a gold-labeled, affinity-purified polyclonal IgG antibody to carbonic anhydrase III, and 2) a conjugate between CAIII and an anti-myoglobin monoclonal IgG antibody, prepared using a heterobifunctional cross-linking reagent. Immobilized at the capture line is another anti-myoglobin monoclonal IgG antibody, recognizing a different epitope on myoglobin than that of the aforementioned conjugate. The assay format may be as described in co-pending application Ser. No. 09 / 130,164, filed Aug. 6, 1998, now U.S. Pat. No. 6,171,870, in which a whole blood sample is applied to the device and red blood cells in the whole blood sample are detained in migration providing...
example 2
Three-Conjugate, Qualitative and Quantitative Assays for Heart Attack
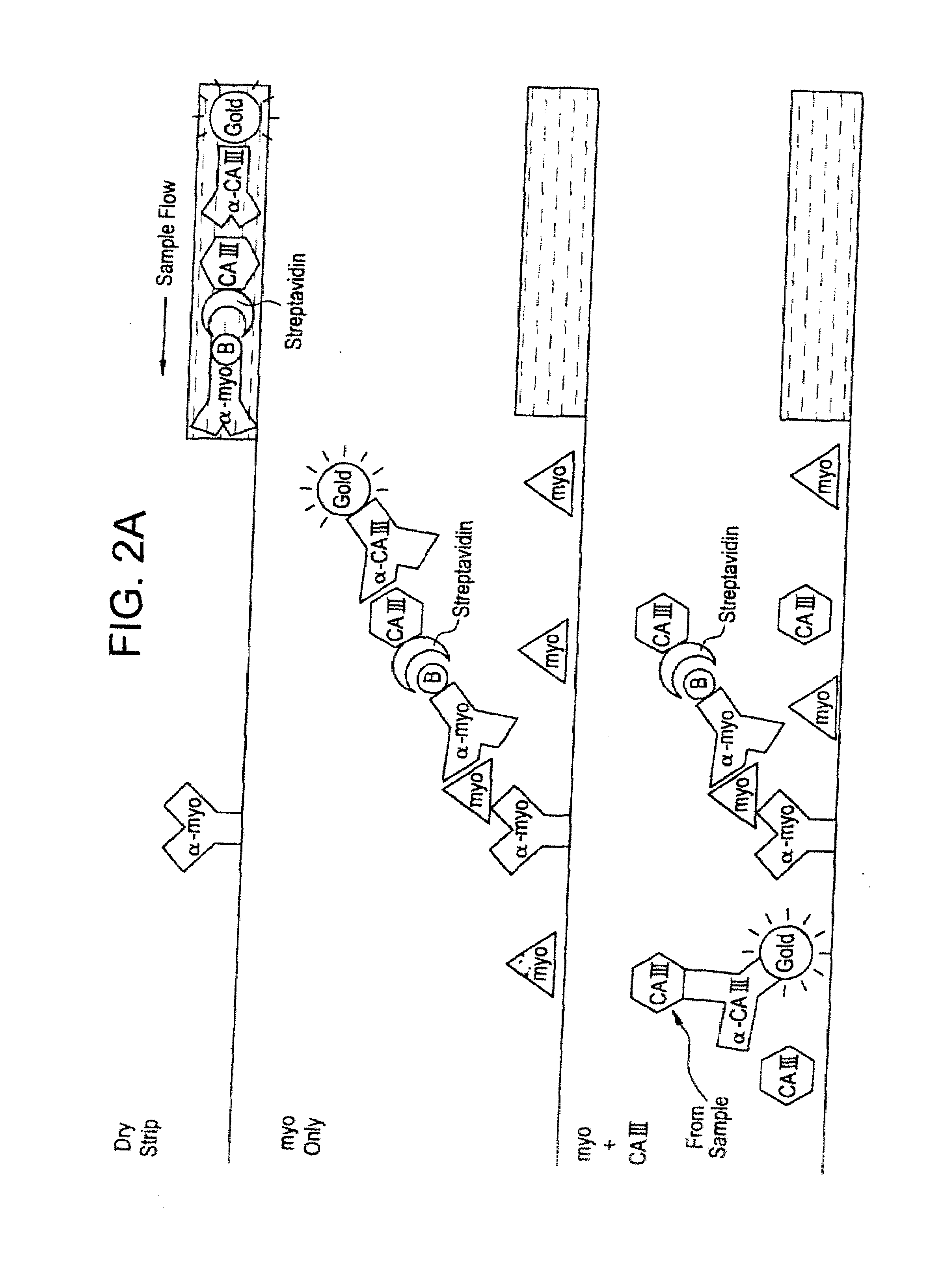
[0192]FIG. 2A illustrates the design and operation of a membrane strip-format assay which is positive for myoglobin only if carbonic anhydrase III is absent. Present in the detection zone of the test strip is an analyte labeling reagent in the form of three mobile reagents: 1) a gold-labeled, affinity-purified polyclonal IgG antibody to carbonic anhydrase III, prepared as described in Example 1 above, 2) a conjugate between carbonic anhydrase III and streptavidin, prepared either by engineering a single-chain polypeptide comprising carbonic anhydrase III and streptavidin, or using a heterobifunctional cross-linking agent to cross-link the members, and 3) a biotinylated anti-myoglobin monoclonal IgG antibody. Immobilized at the capture line is another anti-myoglobin monoclonal IgG antibody, recognizing a different epitope on myoglobin than that in the biotinylated reagent. The assay format may be as described in U.S...
example 3
Measurement of Myoglobin of Cardiac Origin
[0198]Before preparing the reagents and assay format for a homogeneous test to measure the level of myoglobin of cardiac origin, the parameters under which the assay should operate were developed using mathematical models. In this assay, the level of total myoglobin in a sample of blood is subtracted by the level of myoglobin of skeletal origin, the latter determined based on the detection of carbonic anhydrase III, which is co-released with myoglobin from skeletal muscle tissue, but is not released from cardiac tissue. The test parameters are established such that a positive test indicates a sufficiently high amount of myoglobin of cardiac origin is present to diagnose a heart attack. The assay system employed is that described in Example 2, above.
[0199]To establish the cut-off value between a myoglobin-carbonic anhydrase III differential diagnostic of a heart attack versus that indicative of skeletal muscle damage, actual patient data from...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap