Expanded nk cells
a technology of expanded and activated nk cells and cytotoxicity, which is applied in the direction of biocide, plant growth regulators, blood/immune system cells, etc., can solve the problems of inadapting the dose of cells to clinical trials, affecting the clinical effect of autologous nk cells, and affecting the treatment effect of mm patients, etc., to achieve the effect of increasing the effect of administered cells and increasing the cytotoxicity of expanded and activated nk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Ex Vivo Expansion of NK Cells from PBMCs
[0055]Material & Methods: The culture conditions for the expansion of cytotoxic cells have previously been optimized on PBMCs from healthy individuals (Carlens et al., Hum. Immunol. 2001; 62:1092-1098). Briefly, PBMCs were initially thawed and cultured in T25 flasks (TPP, Trasadingen, Switzerland) at a concentration of 0.5×106 cells / ml in CellGro SCGM serum-free medium (CellGenix, Freiburg, Germany) with the addition of 5% human serum (Biowhittaker-Cambrex, Md., USA) and 500 U / ml rhlL-2 (Proleukin®, Chiron Corporation, Emeryville, Calif., USA). For the first 5 days, the medium was further supplemented with anti-CD3 antibody (Orthoclone OKT-3, Ortho Biotech Inc., Raritan, N.J., USA) to a final concentration of 10 ng / ml. On day 5 of culture, the OKT-3-containing medium was washed out, and fresh medium with IL-2 (500 U / ml) and 5% human serum was added. The cultures were then replenished with fresh medium every other day throughout the culture per...
example 2
Flow Cytometry Based Phenotyping of NK Cells and NK Ligands on Multiple Myeloma (MM) Cells
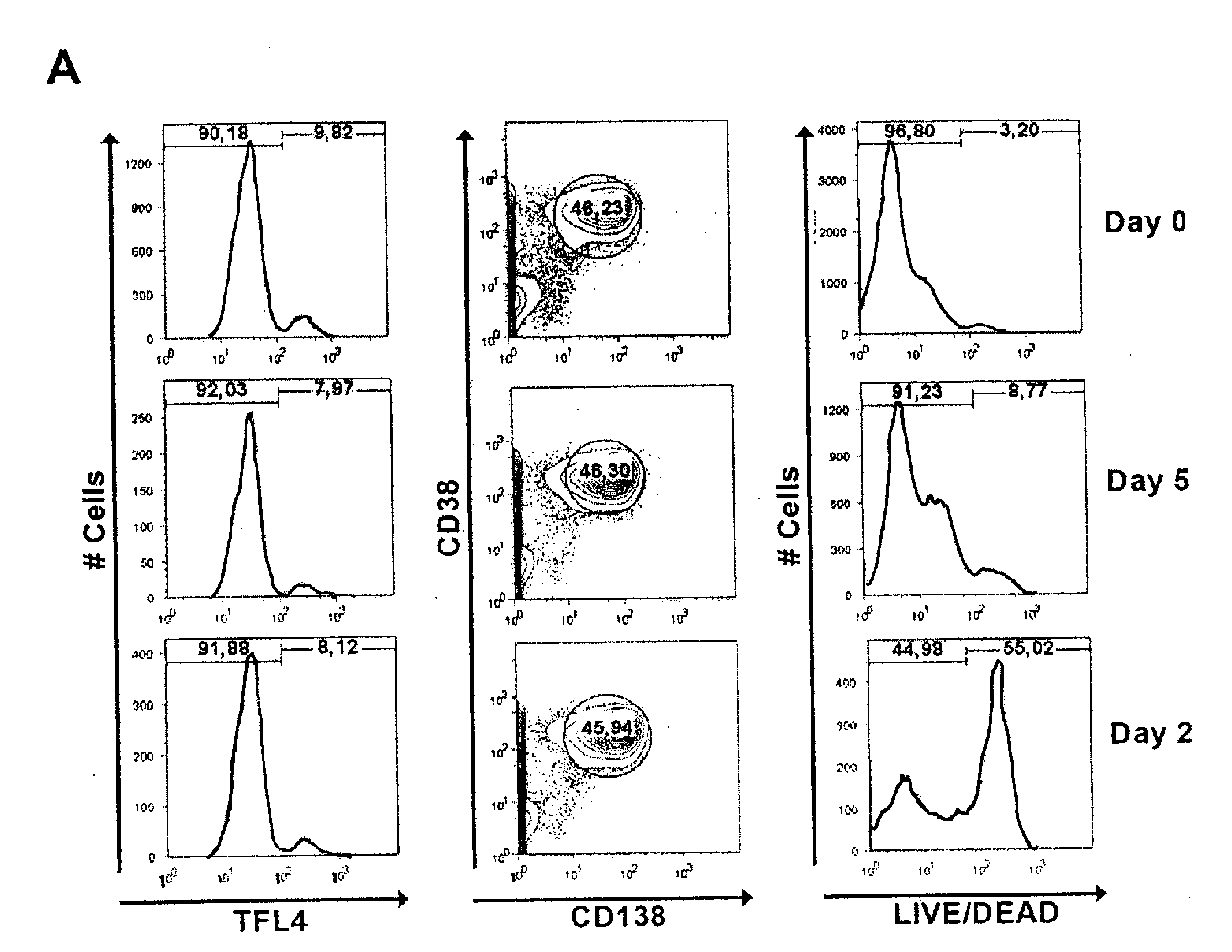
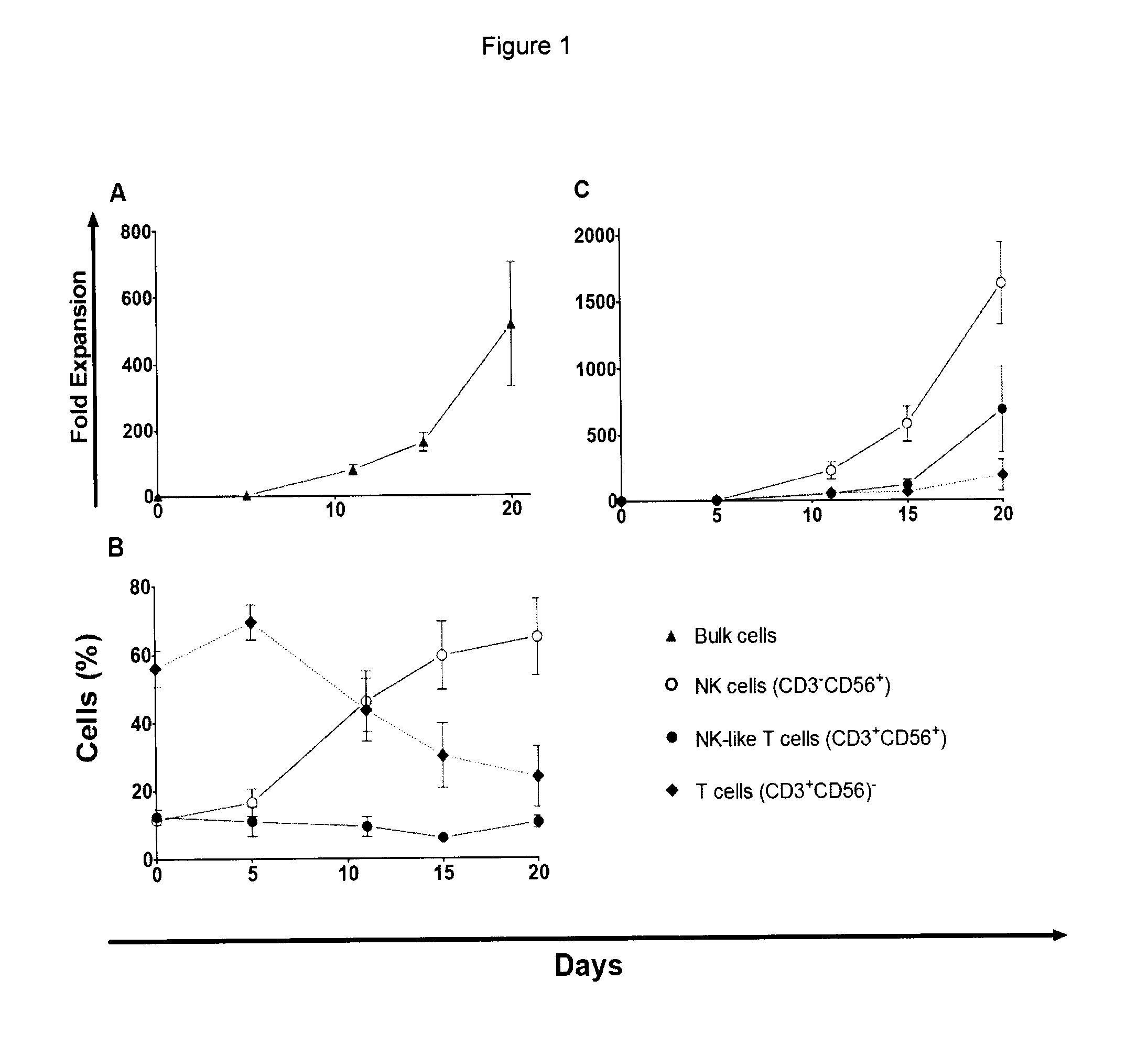
[0057]Material & Methods: The cell phenotype and expansion dynamics of subpopulations were analyzed by flow cytometry on days 0, 5-6, 9-10, 14-15 and 20 of culture using standard procedures with fluorochrome conjugated mAbs against the following surface antigens CD3, CD14, CD38, CD56 and CD138.
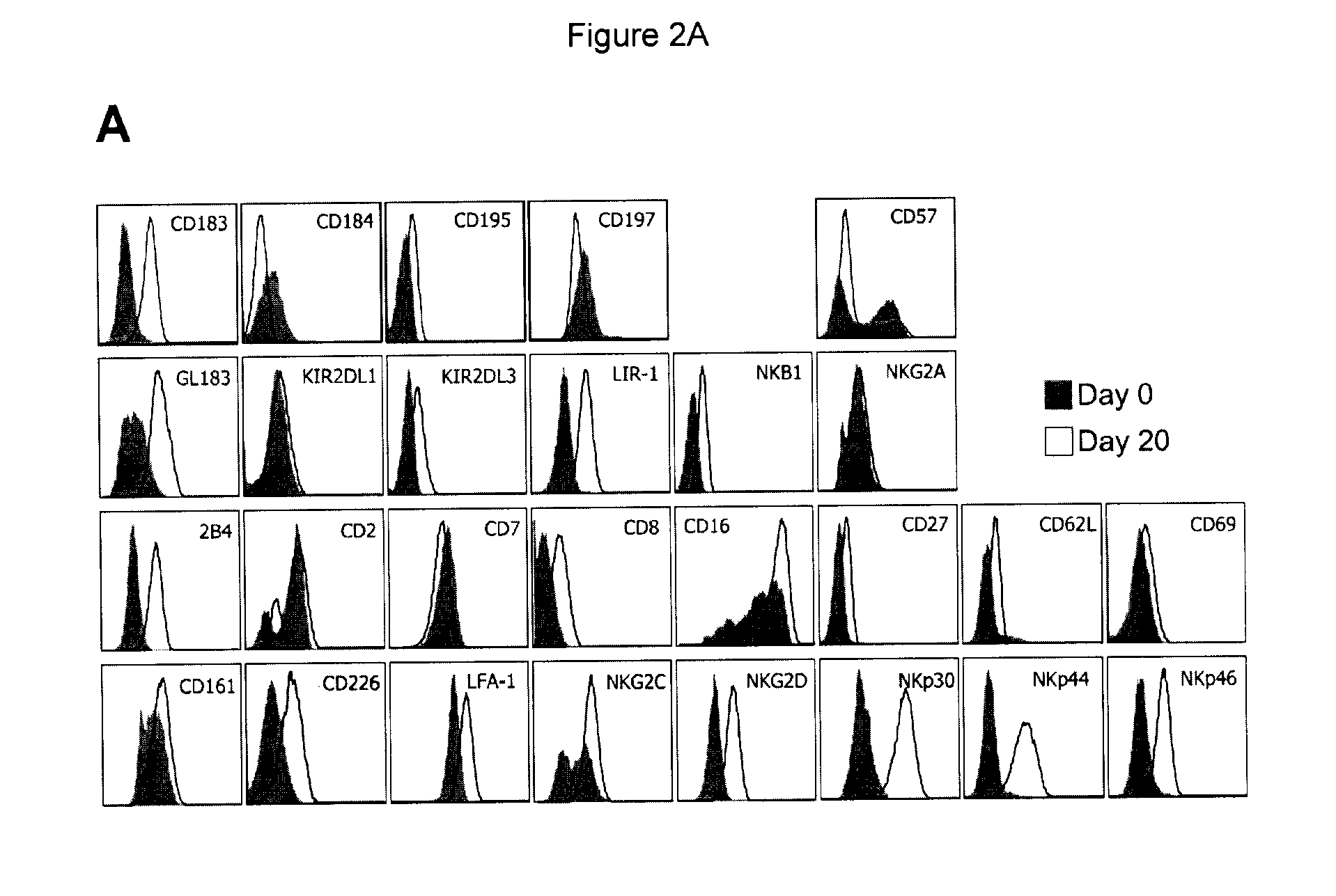
[0058]Day 0 and day 20 cells from all patients were subjected to a more detailed immunophenotypic analysis. To avoid inter-acquisition variability, all frozen samples were simultaneously thawed for a detailed phenotypic characterization of CD56+CD3− (NK) cell subset by flow cytometry. This panel included fluorochrome conjugated mAbs against the following surface antigens: CD2 (RPA-2.10), CD3 (UCHT-1), CD4 (SK3), CD7 (M-T701), CD8 (HIT8a), CD14 (MOP9), CD16 (3G8), CD19 (HIB19), CD25 (M-A251), CD27 (M-T271), CD38 (HIT2), CD56 (B159), CD57 (NK-1), CD161 (DX12), CD183 (3D12), CD184 (12G5), CD195 (2D7 / CCR5), C...
example 3
Evaluation of Cell-Mediated Cytotoxicity
[0062]Material & Methods: The cytotoxic capacity of NK cells before and after expansion was evaluated in vitro with a standard 4 hour 51 Cr-release assay against NK-sensitive K562 cells. Because the 51 Crrelease assay is not suitable for primary MM cells 16-18, a flow cytometry based cell mediated cytotoxicity assay was used.
[0063]For the 51 Cr-release assay, K562 target cells were labelled with 100 μCi of 51Cr for one hour at 37° C., washed twice with PBS, and resuspended in 1 ml of RPMI medium. A total of 3×104 target cells in 100 μl RPMI medium was placed in triplicates into V-bottom 96-well plates and incubated for 4 hours with 100 μl of effector cells at appropriate concentrations to obtain effector:target ratios from 1:3 to 10:1. Aliquots of supernatants were then counted using a Packard Cobra Auto-Gamma 5000 Series Counting System (Meriden, Conn., USA). The percentage of specific 51Cr release was calculated according to the formula: per...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| fluorescent cell counting assay | aaaaa | aaaaa |
| fluorescence intensity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com