Prostate carcinogenesis predictor
a prostate cancer and predictor technology, applied in the direction of instruments, transferases, drug compositions, etc., can solve the problem of achieve the effect of reducing cell proliferation, reducing cell cycle, and maintaining static control of prostate cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PKC-ι is Overexpressed in Malignant or Neoplastic Prostate Tissues
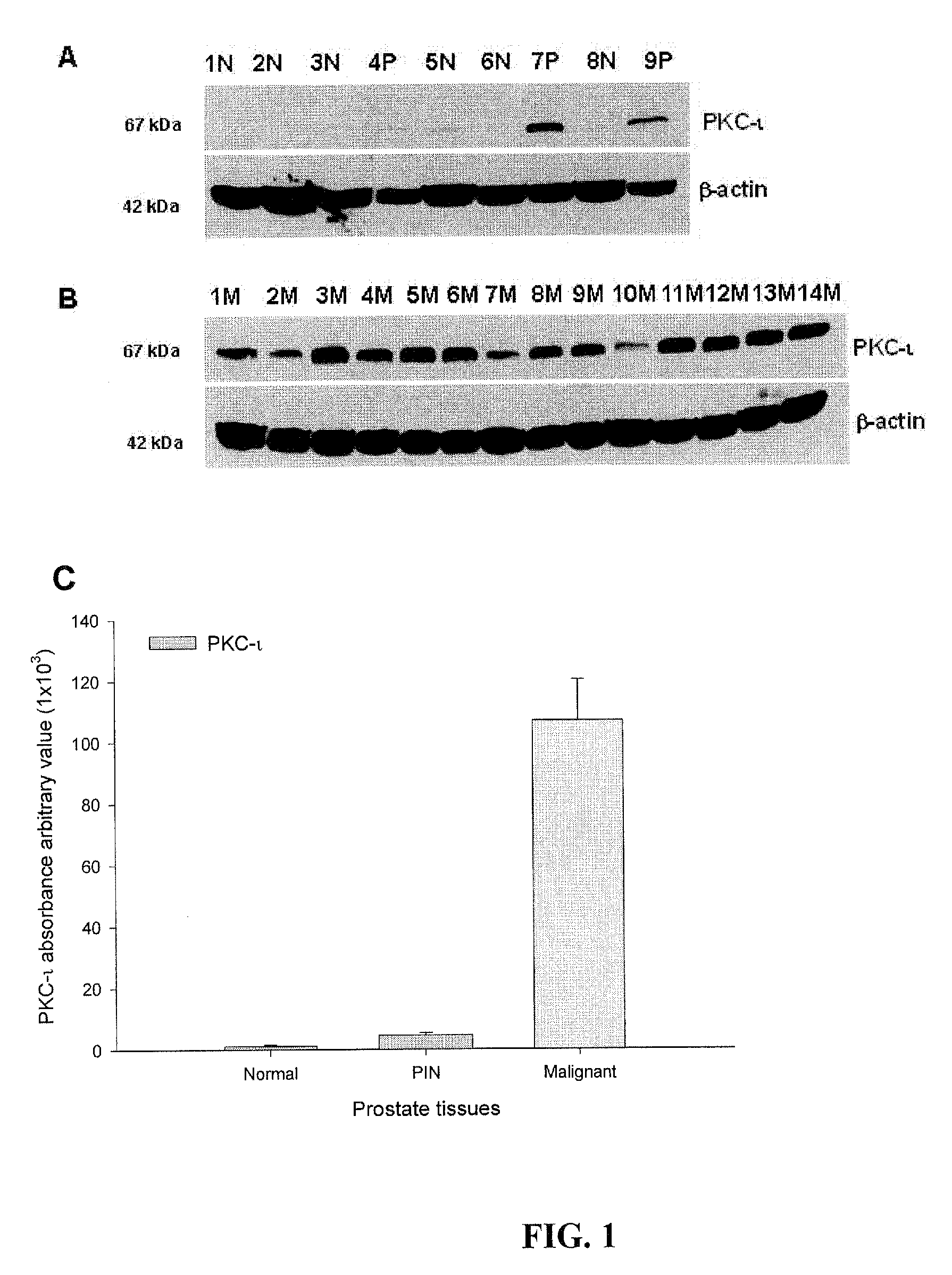
[0359]The relationship between the absence of PKC-ι in BPH tissue and its robust presence in malignant prostate tissue is summarized in Table 1. Western blots probing for PKC-ι in 6 BPH biopsies, 3 PIN, and 7 malignant tumors revealed absence of PKC-ι in BPH (Table 1). By comparison, PKC-ι was robustly present in two PIN and absent in one. PKC-ι was abundant in all malignant prostate tissue. Western blots corresponding to some of the data present in Table 1 are shown in FIGS. 1A and 1B. PKC-α and PKC-ι were identified in Western blots by bands with molecular weights of 80 kD and 67 kD, respectively which corresponded to the immunoreactive signal obtained from U-373MG glioma cells which contain PKC-α and PKC-ι (data not shown). Western blot controls for PKC-α did not show a pattern of expression specific to BPH, PIN or malignant prostate tumors. Control β-actin Western blots showed β-actin immunoreactive bands at a mol...
example 2
Immunoblot Analysis of PKCs in siRNA Treated Prostate Cells
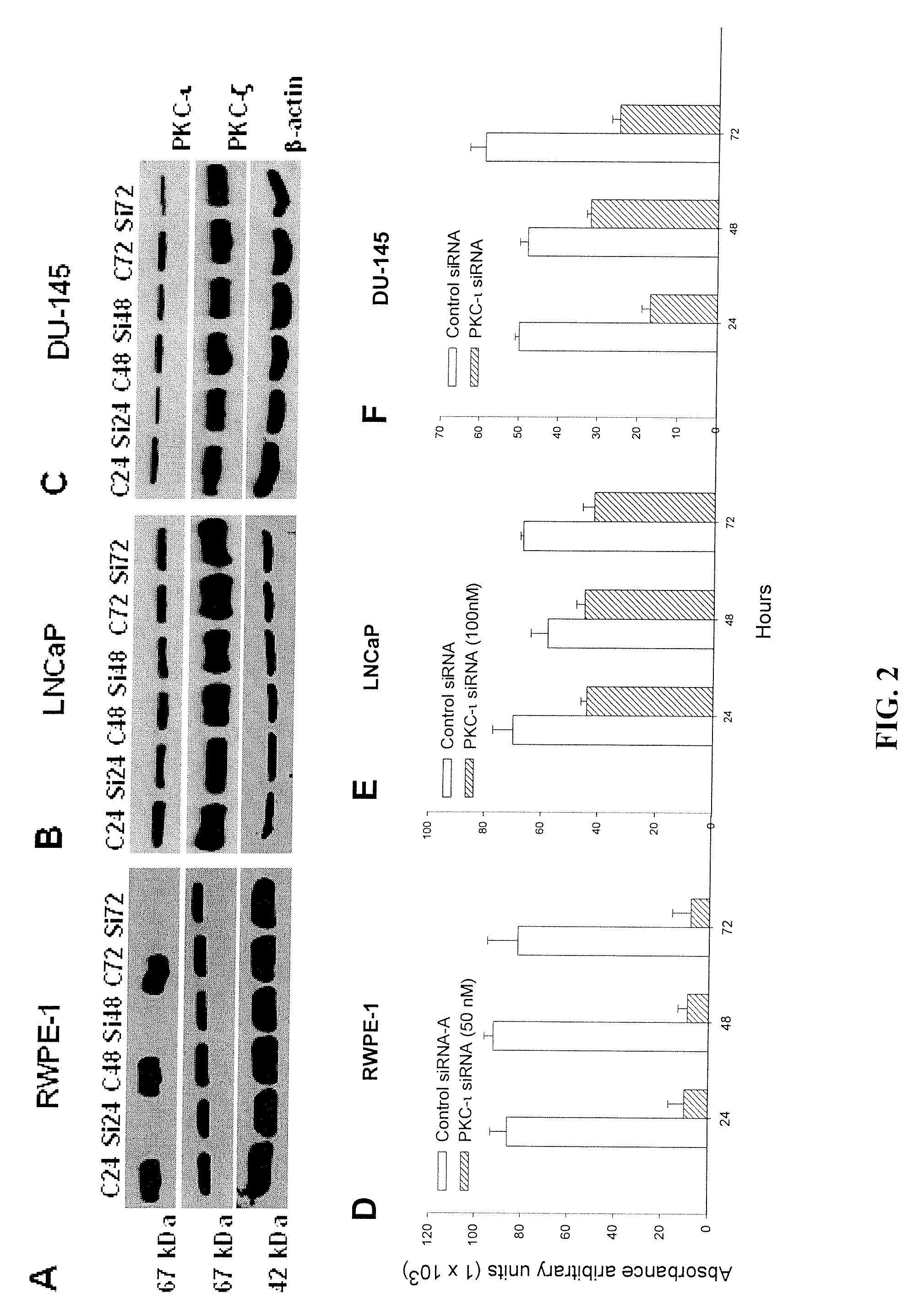
[0362]Immunoblotting of PKC-ι expression shows no effect in control siRNA (See FIG. 2 A-C). After counting the cells (from FIG. 3), the same populations of treated cells were immunoblot for PKC-ι, as described in “Materials and Methods.” A protein concentration of 15 μg was loaded on each lane for each time point and separated by SDS-PAGE and Western blotted with anti-PKC-ι mouse monoclonal or anti-PKC-ζ rabbit polyclonal antibody. Immunoblotting for PKC-ι in PKC-ι siRNA treated RWPE-1 cells showed that expression decreased by 90-92%. Absorbance densitometry (FIGS. 2A and 2D) shows the average of three independent PKC-ι immunoblot for both RWPE-1 cell treatments (p=0.013, 0.005, 0.029). To show specificity, we immunoblotted for PKC-ζ isoforms which is 98% identical to PKC-ι (FIG. 2A, second row).
[0363]There was no or very little PKC-ι present in PKC-ι siRNA treated cells compared to control siRNA. Furthermore, PKC-ι siRNA ha...
example 3
PKC-ι siRNA Treatment Decreases Prostate Cell Viability and Inhibits Cell Cycle Progression
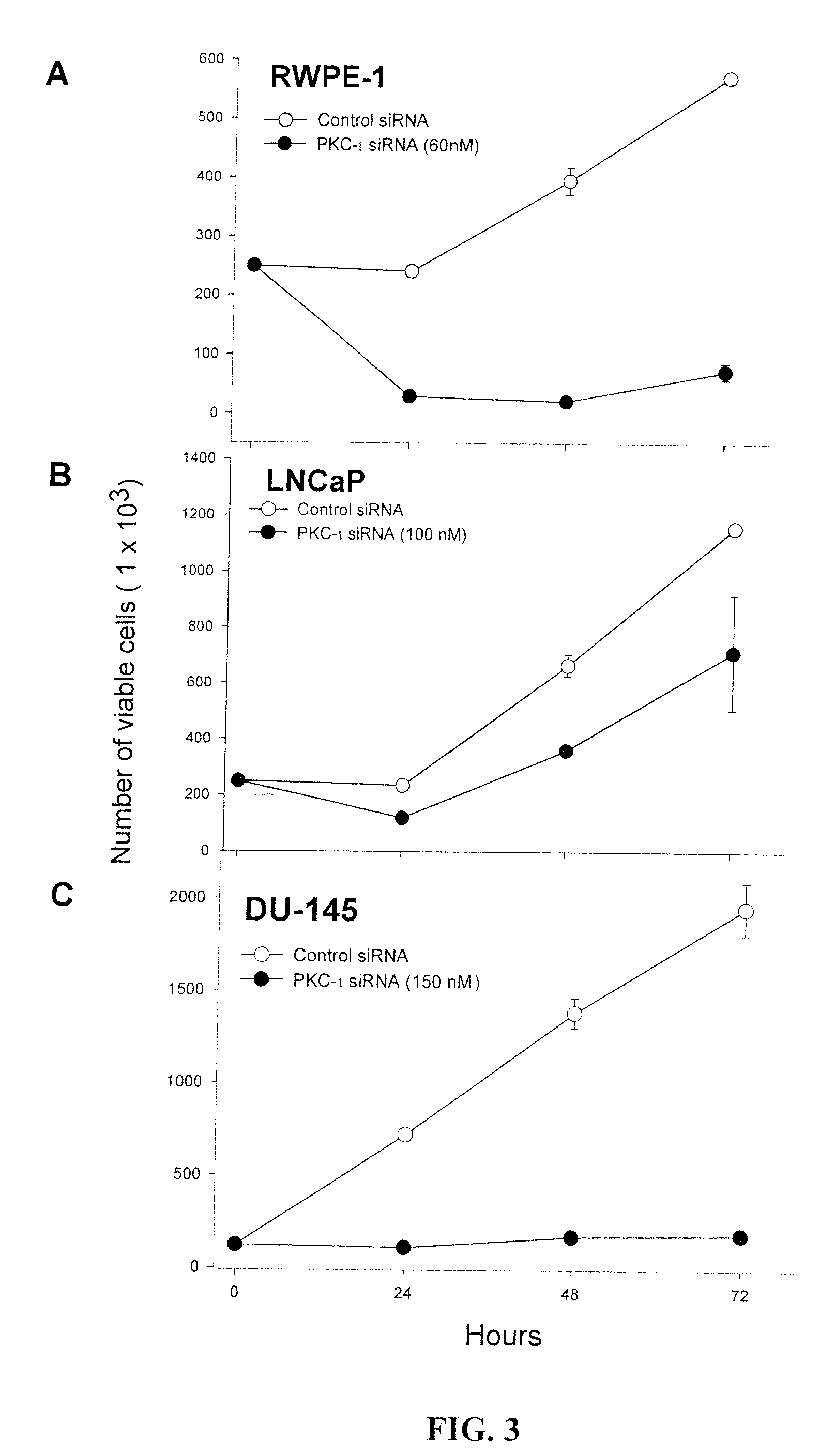
[0365]To provide additional evidence that PKC-ι is required for cell proliferation in RWPE-1 cells, PKC-ι was temporarily inhibited using PKC-ι siRNA. Subconfluent RWPE-1 (A), LNCaP (B) and DU-145 (C) cells (2.5×105) were treated with PKC-ι siRNA (50 nM for RWPE-1, 100 nM for LNCaP and 150 μM for DU-145) complex from 24-72 h as described in “Materials and Methods.” At the indicated time, the viable cells were counted using trypan blue exclusion assay and a hematocyometry. Three independent experiments were performed and the mean of the viable cells and SD were plotted. After titrating PKC-ι siRNA concentrations from 50-100 nM, we used an optimal concentration of PKC-ι siRNA (50 nM) and control siRNA to suppress PKC-ι expression. It was shown that there is a decrease in cell proliferation in PKC-ι siRNA treated cells compared to control siRNA (FIG. 3A-C). Their standard values were as follow: R...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com