Nanoparticulate meloxicam formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
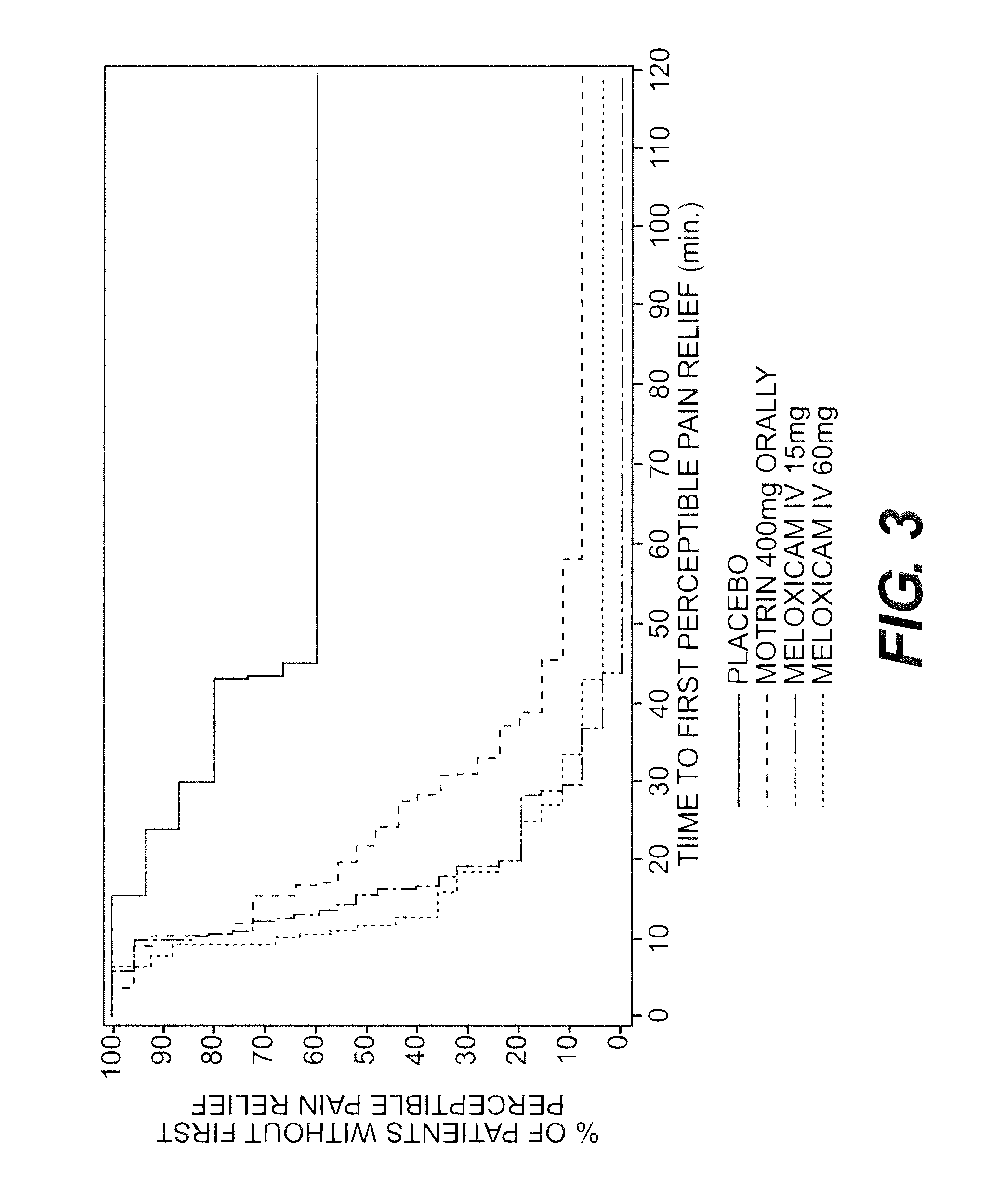
[0127]This example identifies and exemplary method to prepare a nanoparticulate meloxicam dispersion suitable for injection.
[0128]A slurry of 20% (w / w) meloxicam and 4% (w / w) polyvinyl pyrrolidone were milled in a NanoMill® milling system (Elan Drug Delivery, Inc., King of Prussia, Pa.; see e.g., U.S. Pat. No. 6,431,478 for “Small Scale Mill”).
example 2
[0129]Aqueous dispersions of 5 wt. % meloxicam and 1 wt. % stabilizer (see Table 1, below) were charged into a NanoMill® milling system (Elan Drug Delivery, Inc., King of Prussia, Pa.; see e.g., U.S. Pat. No. 6,431,478 for “Small Scale Mill”).
[0130]Particle size analysis of the resultant milled dispersions was performed using a Horiba LA-910 particle size analyzer (Horiba Instruments, Irvine, Calif.). The results are shown below in Table 1. In the table below, the value for D50 is the particle size below which 50% of the active agent particles fall. Similarly, D90 is the particle size below which 90% of the active agent particles fall.
TABLE 1MeanD50D90Stabilizer(nm)(nm)(nm)Optical Microscopy*poloxamer 188133110226Stablepoloxamer 388129108219Stablepolyvinylpyrrolidone k-129890125Stablepolyvinylpyrrolidone k-179895135StablePolysorbate 80227227322StableSodium Deoxycholate119101198StableLecithin190169271Mild aggregationat initialLysozyme9589117Moderate aggregationat initial;Stable at 24...
example 3
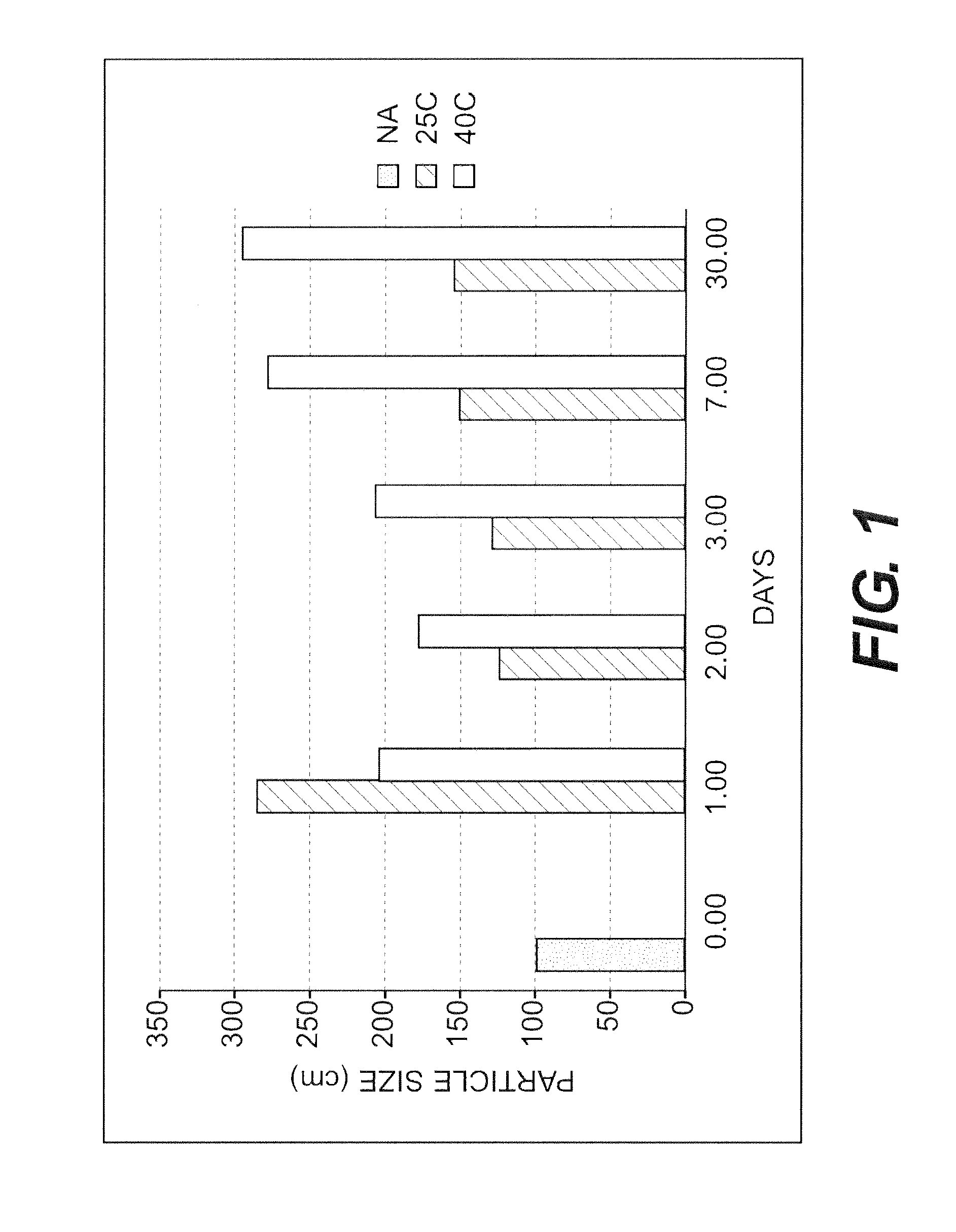
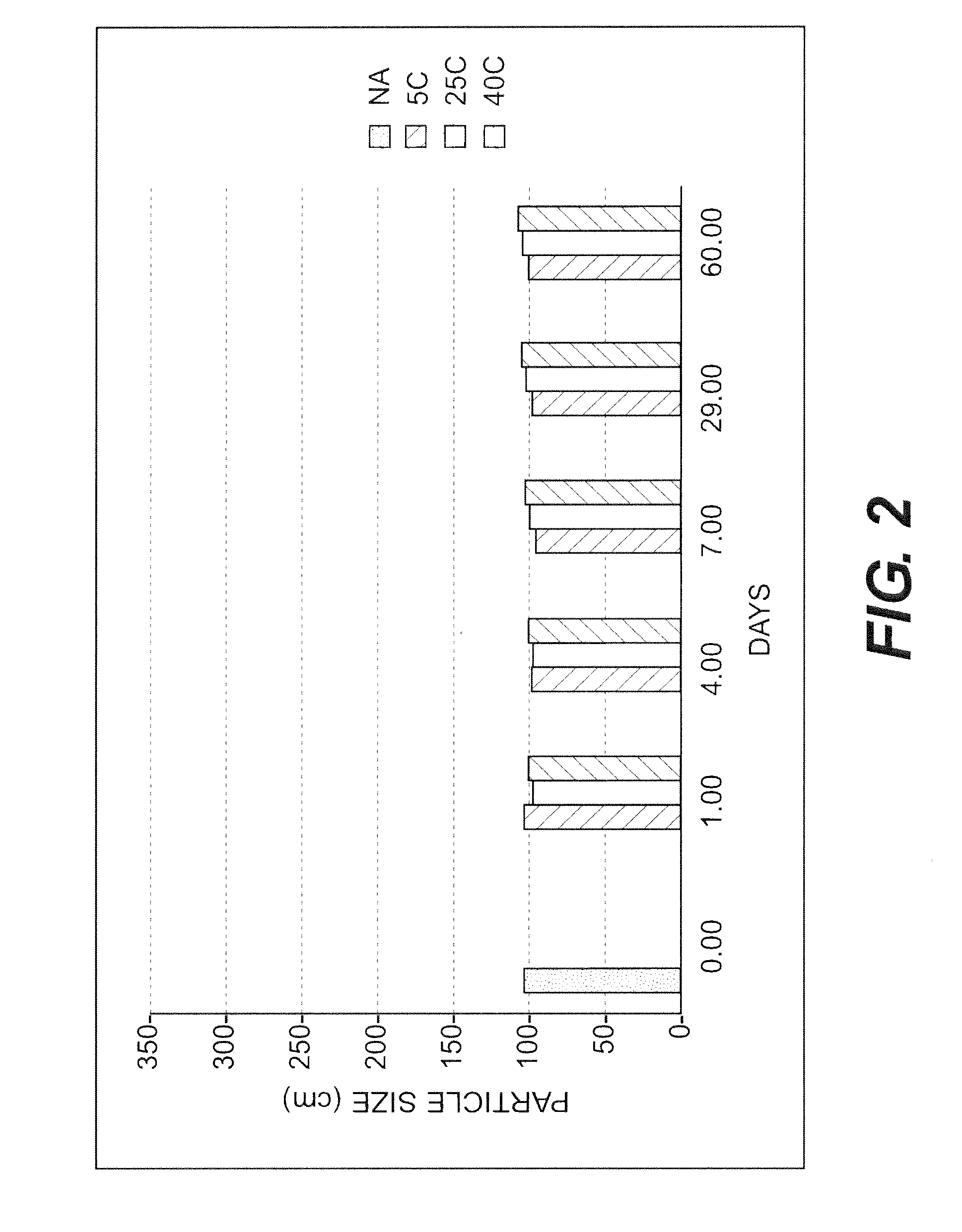
[0133]The purpose of this example was to test the stability of a nanoparticulate meloxicam formulation comprising mannitol.
[0134]A slurry of 10% (w / w) meloxicam, 2.5% (w / w) polyvinylpyrrolidone, 0.75% (w / w) NaDOC and 10% (w / w) mannitol was milled to obtain a nanoparticulate dispersion of meloxicam. The nanoparticulate meloxicam dispersion was diluted to 5% meloxicam, 1.25% polyvinylpyrrolidone, 0.375% NaDOC, 5% mannitol and 15% sucrose with a 30% sucrose solution. The formulation was stored at 5° C. for 3 months. The resulting nanoparticulate meloxicam formulation did not show any significant particle agglomeration or aggregation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com