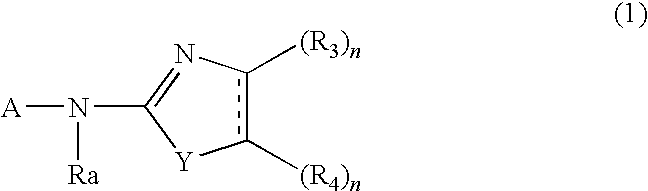
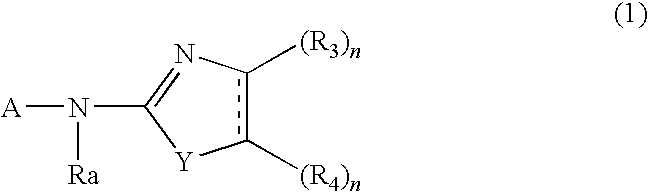
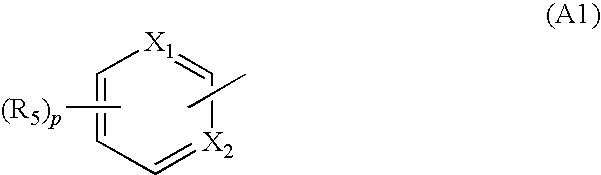Whitening Agent And Skin External Preparation
a technology of external preparation and whitening agent, which is applied in the direction of biocide, heterocyclic compound active ingredients, drug compositions, etc., can solve the problems of insufficient safety, unreachable literature, and insufficient effect of whitening agent, and achieve excellent inhibitory action on melanin production and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1-1
Synthesis of 4-(5,5-dimethyl-4,5-dihydrothiazol-2-yl amino)phenol (Compound 41)
[0103]To p-anisidine (0.60 g, 4.86 mmol) and methanol (4.0 mL) in a 50 mL recovery flask was added methallyl isothiocyanate (0.50 g, 4.42 mmol) dropwise at room temperature, and then the resulting mixture was stirred for 12 hours at room temperature. Upon completion of the reaction, the mixture was extracted with ethyl acetate once. The organic phase thus obtained was washed with saturated brine and then dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure and the residue was recrystallized from a mixed solvent of ethyl acetate and hexane to give 0.79 g of 1-(4-methoxyphenyl)-3-(2- methylallyl)thiourea (yield 76%).
[0104]1-(4-Methoxyphenyl)-3-(2-methylallyl)thiourea (0.50 g, 2.11 mmol) and 35% hydrochloric acid (5.0 mL) were added in a pressure resistant reaction container, and the resulting mixture was stirred at 140° C. for five hours in the sealed container. Upon ...
synthesis example 1-2
Synthesis of (hetero)arylaminothiazolines
[0105](Hetero)arylaminothiazolines as shown in Table 6 were each synthesized in the same manner as in Synthesis Example 1-1 except that raw material A was used instead of anisidine.
TABLE 6No.StructureRaw material ANMRYield41p-anisidine1H-NMR (DMSO-d6): 1.46 (6 H, s), 3.51 (2 H, br-s), 6.61 (2 H, dd), 7.07 (2 H, br-s), 8.67 (2 H, br-s)40%42m-anisidine1H-NMR (DMSO-d6): 1.47 (6 H, s), 3.64 (2 H, br-s), 6.31 (1 H, d), 6.83 (2 H, br-s), 6.97 (1 H, t), 9.17 (2 H, br-s)34%383-aminopyridine1H-NMR (DMSO-d6): 1.49 (6 H, s), 3.53 (2 H, br-s), 7.23 (1 H, dd), 7.66 (1 H, br-s), 8.12 (1 H, dd), 8.35 (1 H, br-s), 9.10 (1 H, br-s)56%
synthesis example 2
Synthesis of 4-(pyridin-2-yl)-N-o-toluylthiazol-2-amine (Compound 2)
[0106]To 975 mL of methanol were added triethylamine (14.0 g, 137 mmol), o-toluylthiourea (11.54 g, 69.4 mmol), and 2-(bromoacetyl)pyridine hydrobromide (19.5 g, 69.4 mmol), and the resulting mixture was stirred for 15 hours at room temperature. Upon completion of the reaction, 1,950 mL of water was added to the mixture and precipitated crystals were collected by filtration. The solid thus collected was crystallized from a mixed solvent of water and methanol to give the title compound (14.0 g, 76%).
[0107]1H-NMR (DMSO-d6): 2.30(3H, s), 7.01-7.04(1H, m), 7.22-7.30(3H, m), 7.46(1H, s), 7.82-7.91(2H, m), 7.97(1H, d), 8.56(1H, d), 9.32(1H, s)
PUM
| Property | Measurement | Unit |
|---|---|---|
| excitation wavelength | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
| Ra | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap