Methods and compositions related to targeting monoacylglycerol lipase
a monoacylglycerol and composition technology, applied in drug compositions, metabolic disorders, cardiovascular disorders, etc., can solve the problems of threatening many people's life and health, malignant tumors (cancer), and none of the previous studies have specifically examined the role of magl, so as to improve the inhibitory activity of magl, improve the effect of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Development of Selective Inhibitors of MAGL
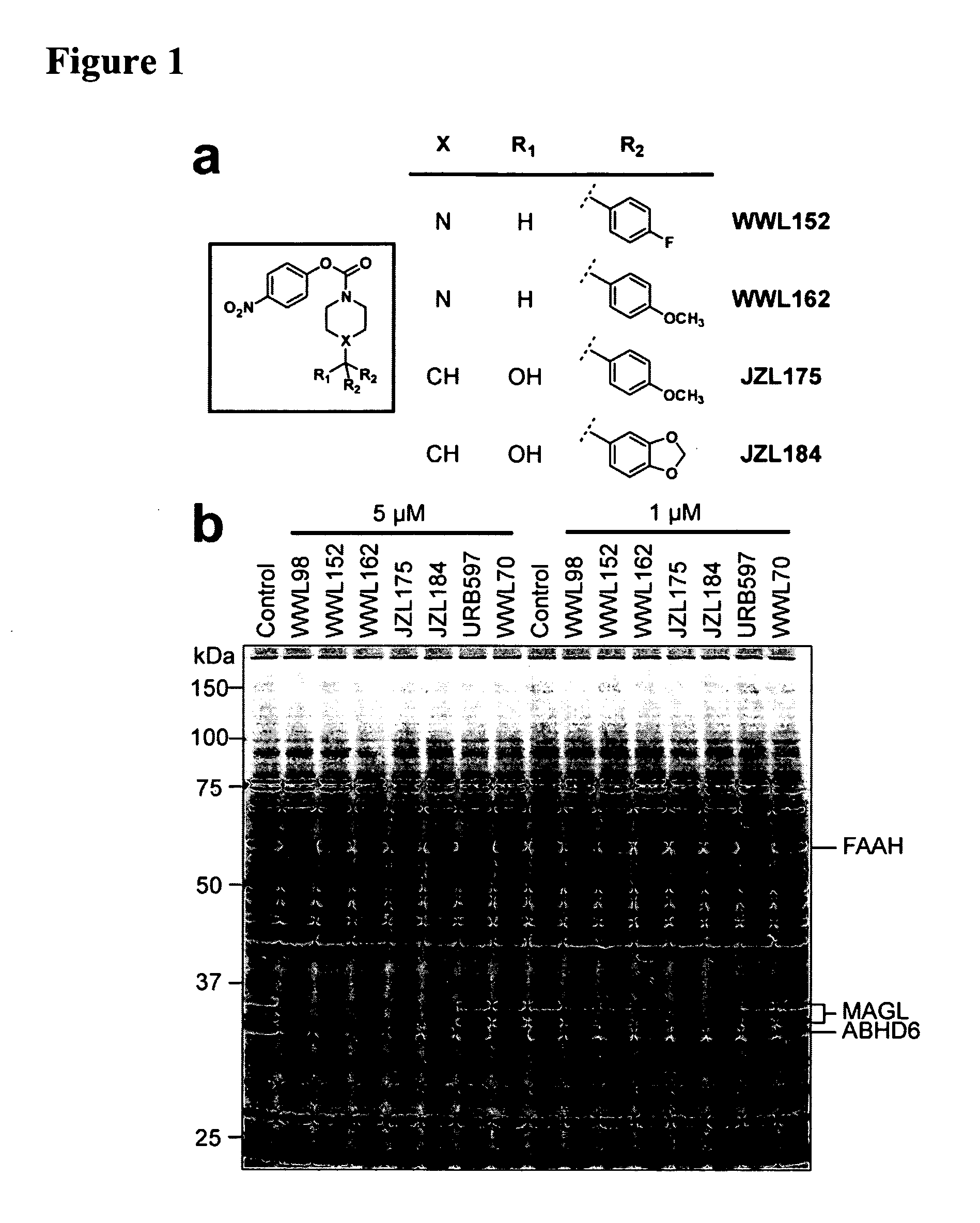
[0102]The pursuit of selective inhibitors for serine hydrolases has the potential to benefit from multiple features special to this enzyme class. First, serine hydrolases are susceptible to covalent inactivation by specific chemical groups that show little or no cross-reactivity with other enzyme classes. Principal among these reactive chemotypes is the carbamate, which has been identified as a privileged scaffold for the design of selective, irreversible inhibitors of serine hydrolases owing to its tempered electrophilicity and hydrolytic stability following covalent reaction (carbamylation) with the conserved serine nucleophile of these enzymes. Second, the functional state of serine hydrolases can be collectively profiled in native biological systems using activity-based protein profiling (ABPP) methods (Cravat et al., Annu Rev Biochem 77:383-414, 2008). ABPP of serine hydrolases uses reporter-tagged fluorophosphonates (FPs), which serve...
example 2
Activity of MAGL Selective Inhibitors in Brain Proteomes
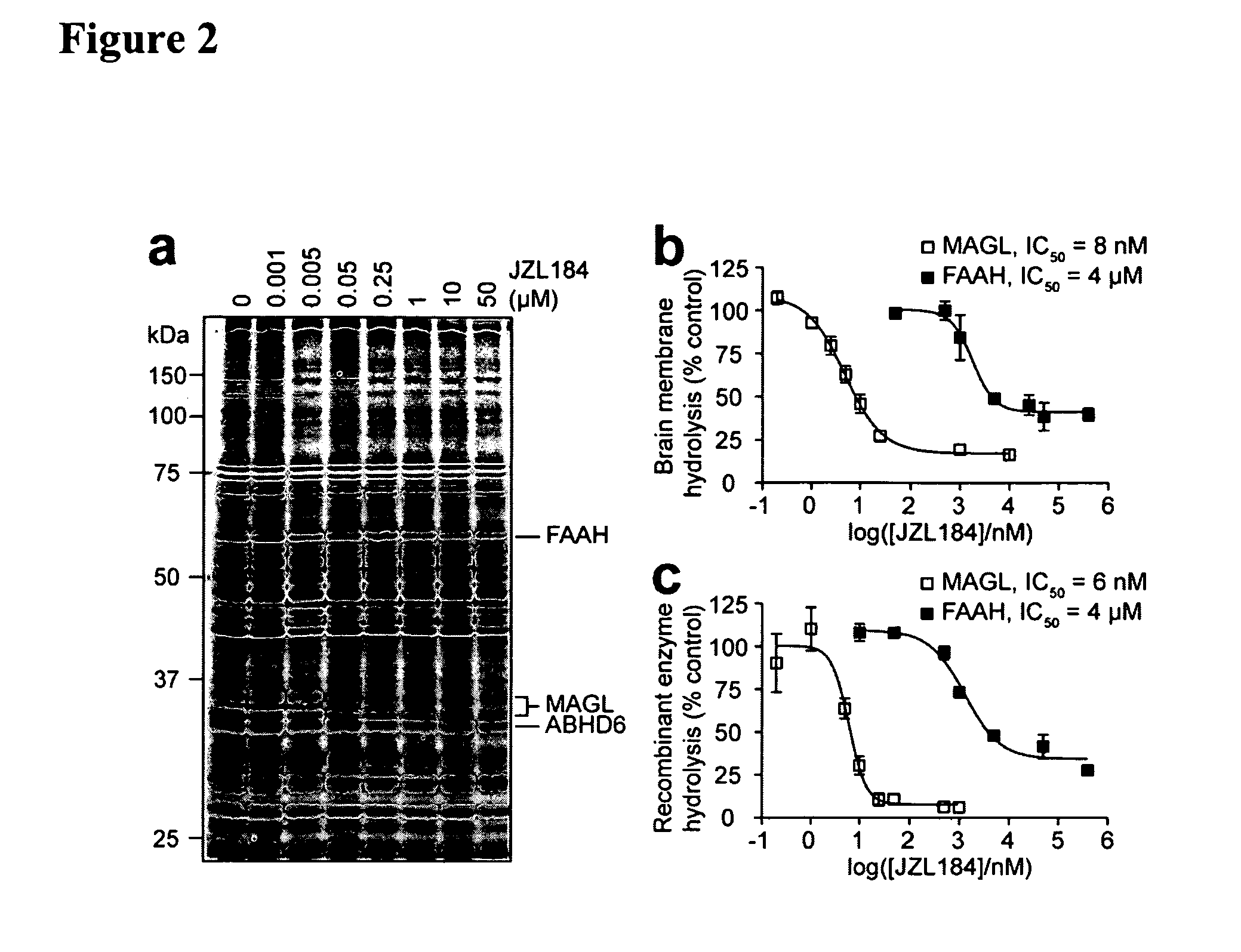
[0104]Near-complete blockade of MAGL activity was observed by competitive ABPP following a 30 min preincubation of a mouse brain membrane proteome with as low as 50 nM JZL184 (FIG. 2A). In contrast, inhibition of other enzymes (FAAH, ABHD6) was not observed until much higher concentrations of JZL184 (10 μM). Substrate assays confirmed this degree of selectivity, as JZL184 displayed IC50 values of 8 nM and 4 μM for blockade of 2-AG and oleamide (FAAH substrate) hydrolysis in brain membranes, respectively (FIG. 2B). Comparable inhibitory effects were observed with recombinant MAGL and FAAH when expressed in COS7 cells (FIG. 2C). Interestingly, brain membranes maintained a residual ˜15% 2-AG hydrolysis activity even at the highest concentrations of JZL184 tested, likely reflecting the contribution of other 2-AG hydrolases that are insensitive to JZL184. JZL184 displayed time-dependent inhibition of MAGL (FIG. 7), indicative of a c...
example 3
In Vivo Activity of MGCL Selective Inhibitors
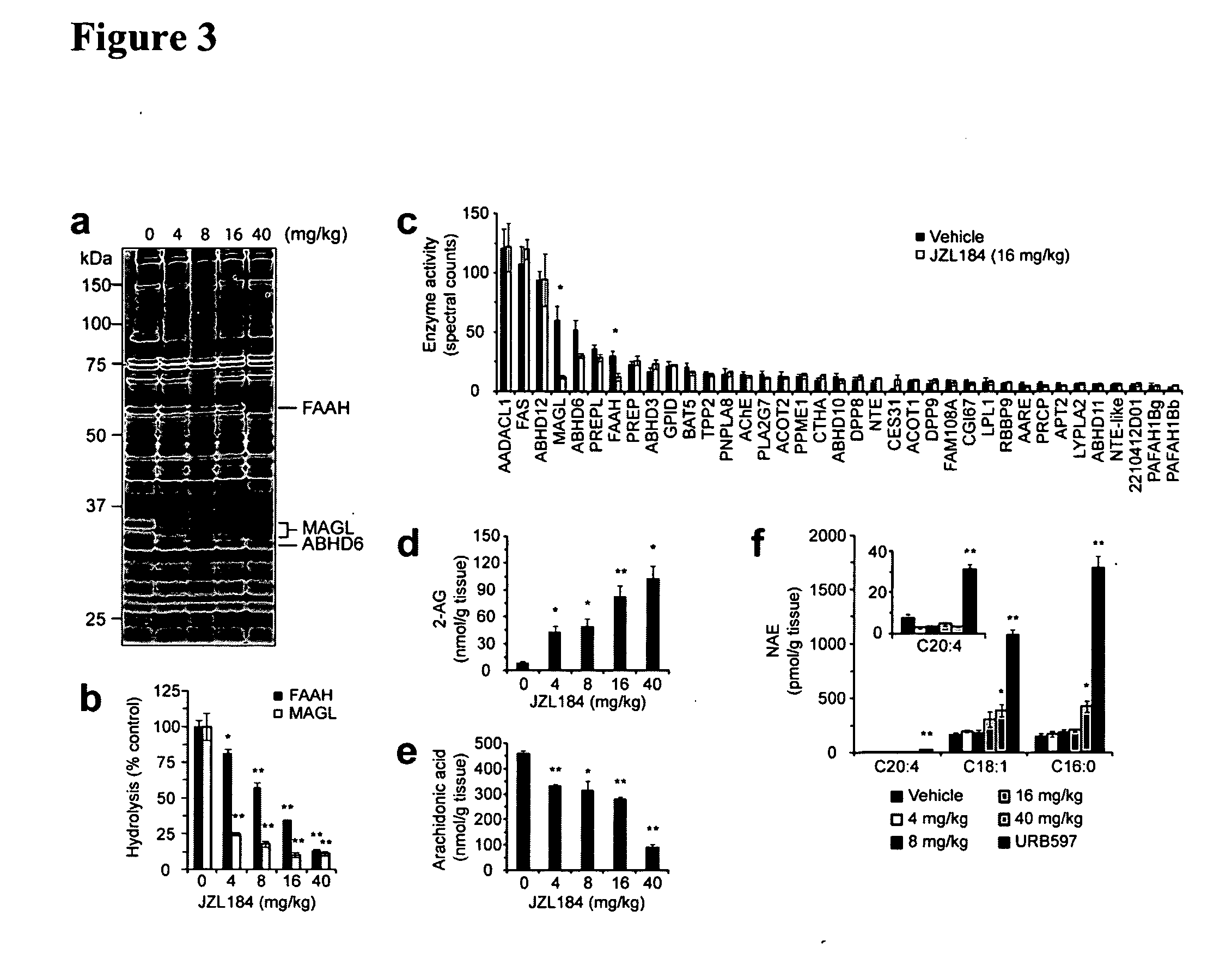
[0105]To assess the ability of JZL184 to block MAGL in vivo, male C57B1 / 6 mice were administered JZL184 (4-40 mg / kg, i.p.) and sacrificed after 4 hrs for analysis. At the lowest dose of JZL184 tested (4 mg / kg), competitive ABPP of brain membrane proteomes revealed 75% MAGL inactivation with minimal effects (<20% inhibition) on other brain serine hydrolases, including FAAH (FIG. 3A). These data were also confirmed for MAGL and FAAH by substrate assays (FIG. 3B). Residual brain 2-AG hydrolysis activity could be further reduced from 25% to 15% of control values by increasing the dose of JZL184 from 4 to 16 mg / kg (FIG. 3B), which correlated with a near-complete blockade of MAGL activity as determined by competitive ABPP (FIG. 3A). FAAH was also inhibited in a dose-dependent manner, but even at 16 mg / kg of JZL184, a substantial fraction of FAAH activity (˜35%) remained intact as determined by competitive ABPP (FIG. 3A) or substrate (FIG. 3B) a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tumor growth time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com