Apparatus method and for intelligent electronic medical devices
a technology of intelligent electronic medical devices and apparatus, applied in the field of apparatus and electronic medical devices for measuring lung performance, can solve the problems of not seeing widespread clinical use of these devices, unable to meet the needs of patients, and physicians do not have the time to review large volumes of raw data, so as to facilitate the improvement of day-to-day asthma control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
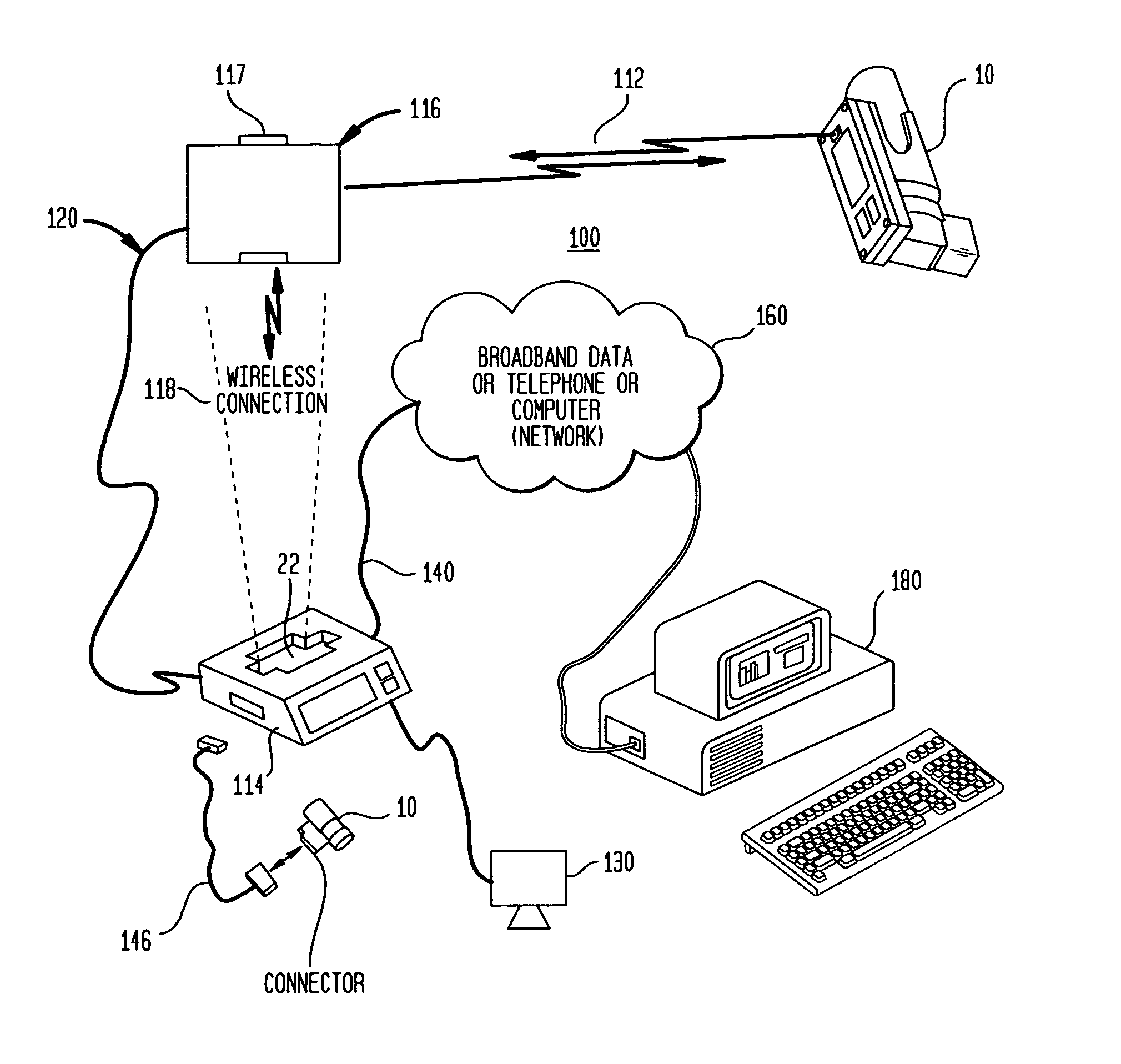
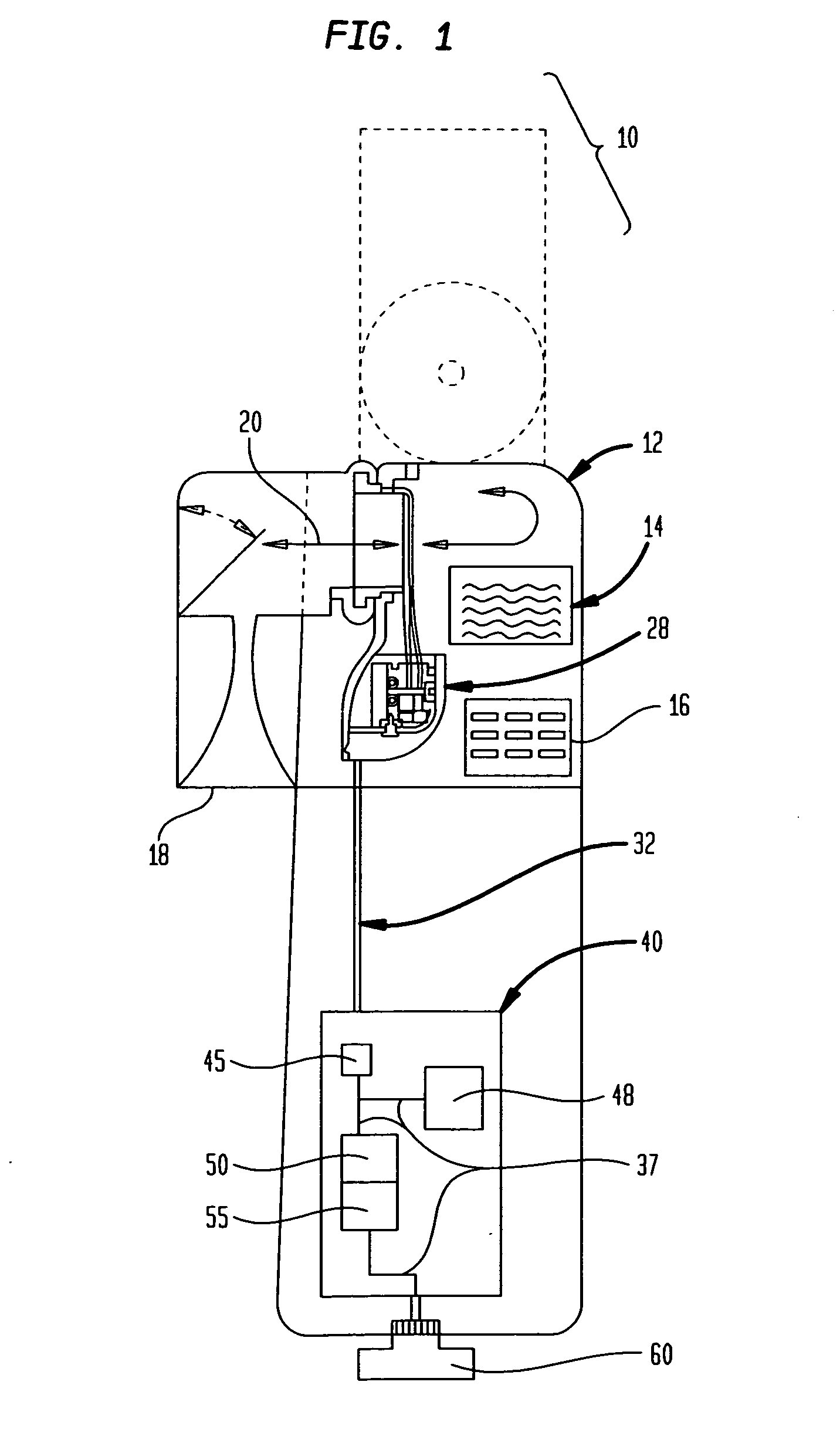
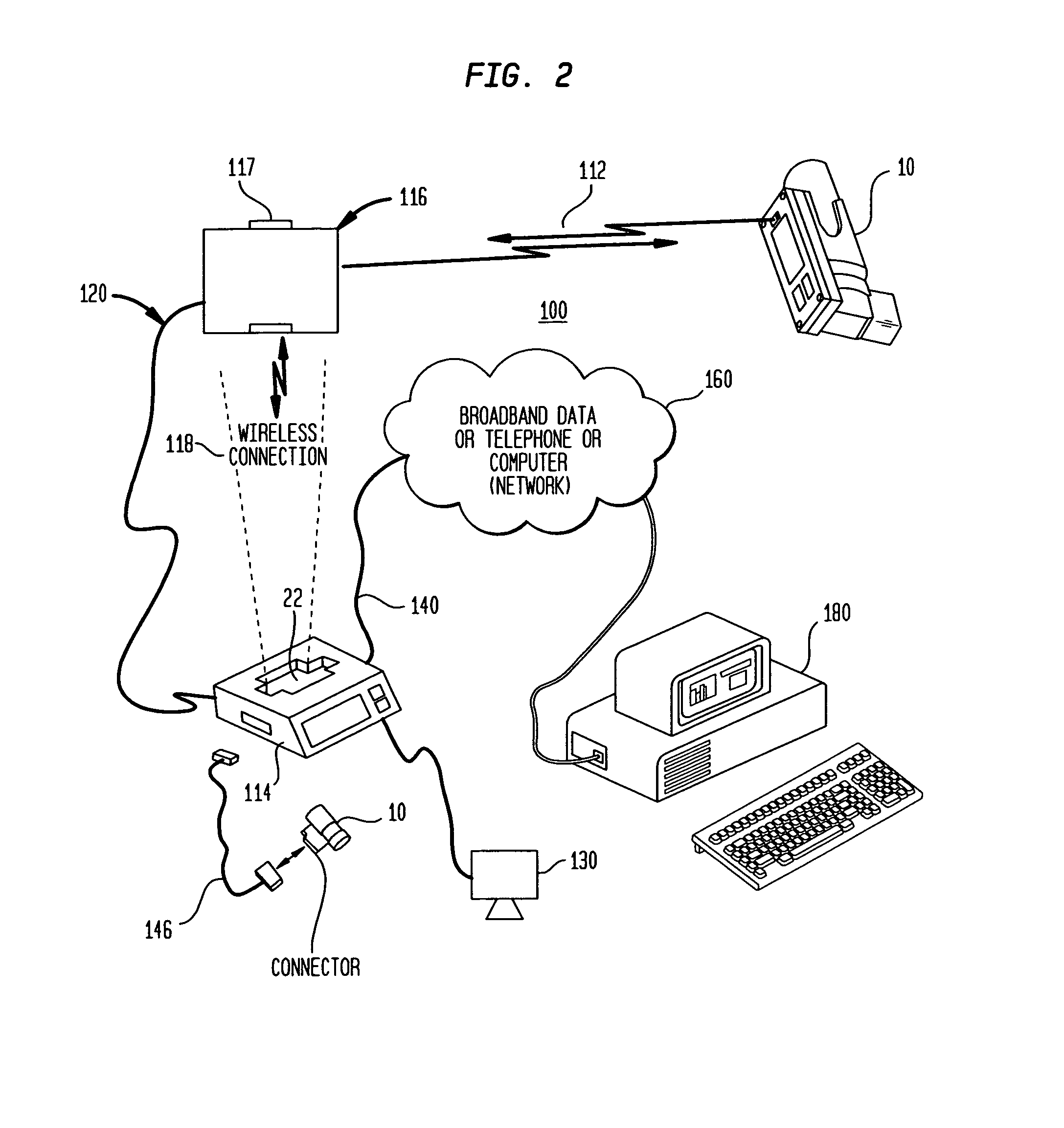
[0090]FIG. 1 illustrates an electronically enhanced peak flow meter 10 (EEPFM) according to an exemplary embodiment of the present invention. The EEPFM accepts a user's respiratory input at opening 18, and channels it along pathway 20 to transducer 28. Transducer 28 converts the mechanical respiratory input into electronic from and transmits the corresponding electronic signals over communications bus 32 to electronics module 40. EEPFM 10 may also include display 14 for providing visual indications and results of a respiratory measurement and provide prompts to the patient during the use of the device. Finally, key entry pad 16 is provided on the EEPFM housing 12 for entering certain data associated with the respiratory testing such as, for example, date, time, or trial number.
[0091]Electronics module 40 is housed within EEPFM 10 and includes essential components according to one embodiment of the present invention. Electronics module 40 is coupled to communications bus 32 via its o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com