Methods and Compositions for Hematoxylin and Eosin Staining
a technology of hematoxylin and eosin, applied in the field of methods and compositions for hematoxylin and eosin staining, can solve the problems of undesirable carry-over, affecting the functionality affecting the flow rate of downstream staining reagents, so as to reduce the number of slides, mitigate the effects of solution carry-over, and enhance the number of slides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
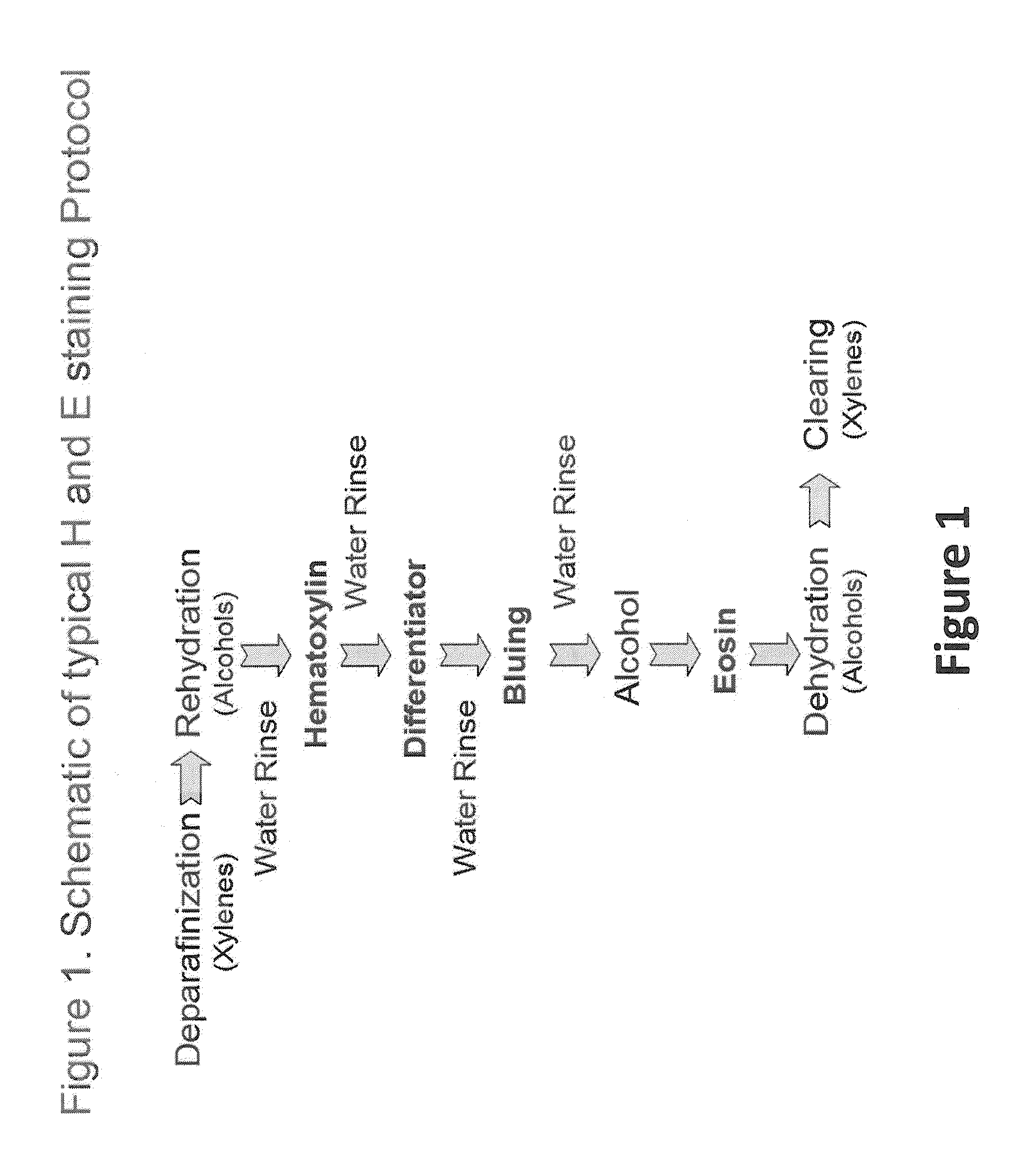
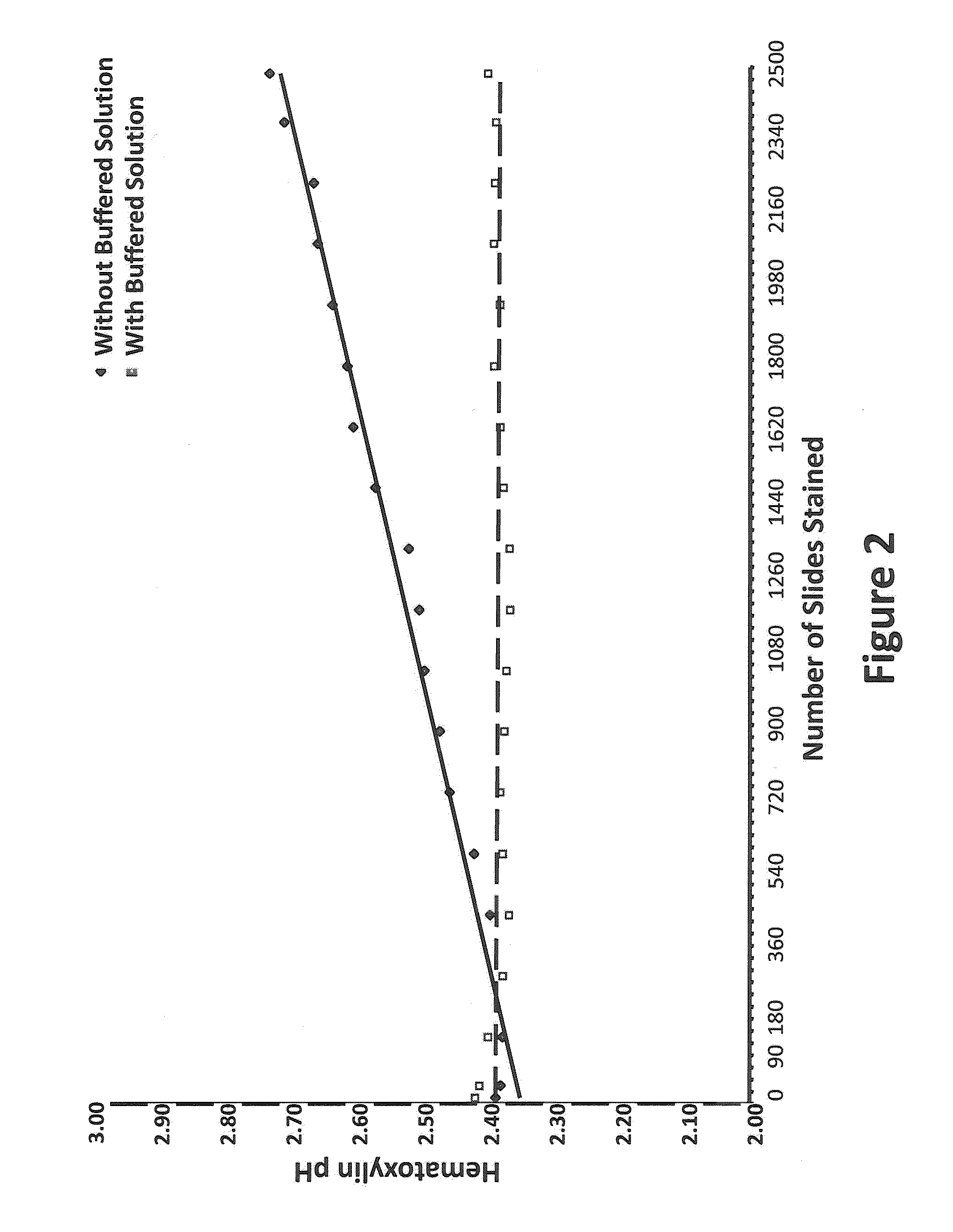
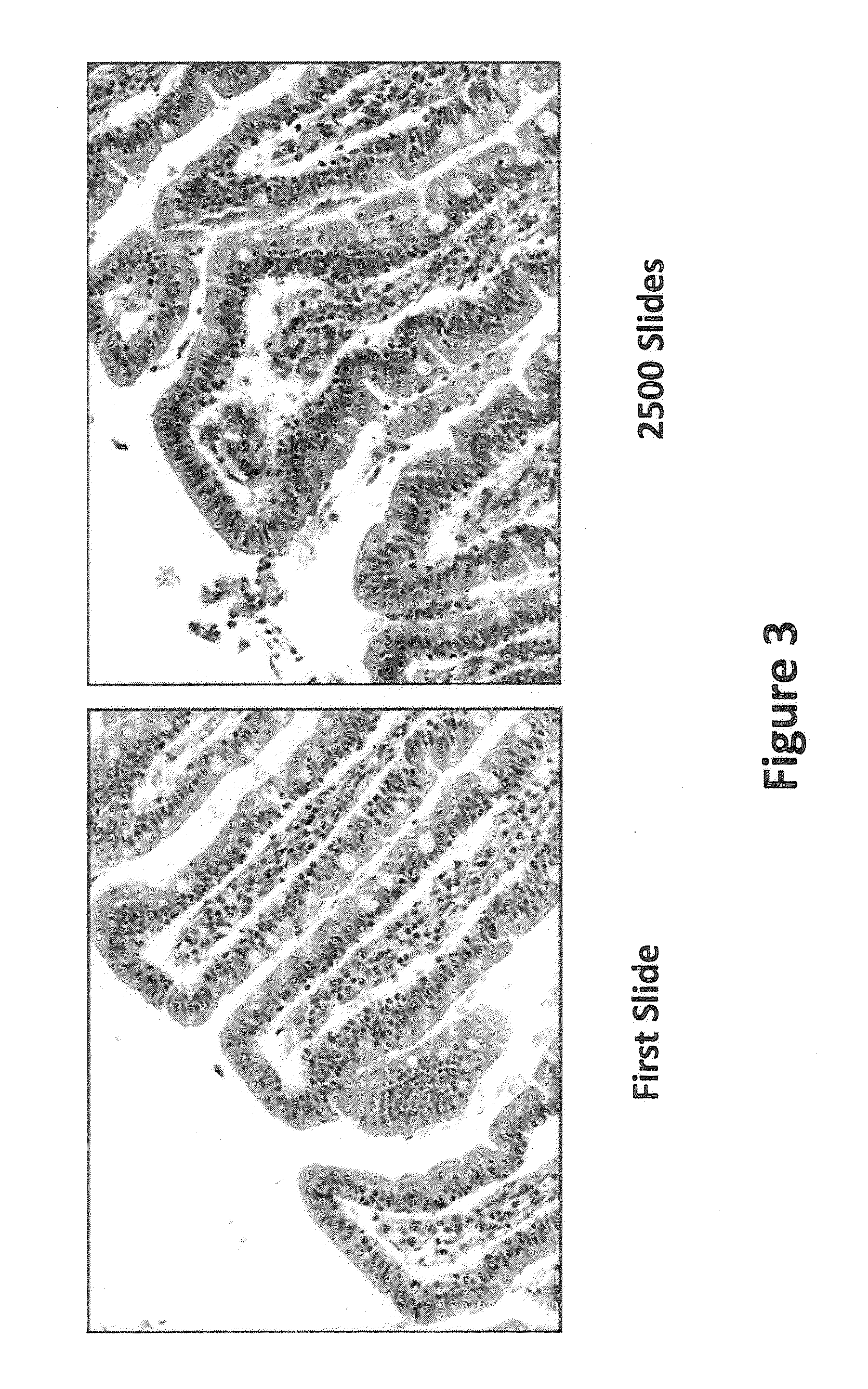
[0046]All staining was performed on either the LEICA® ST-5010 AUTOSTAINER™ or the LEICA® ST-5020 MULTISTAINER™ utilizing a standard H and E program as outlined in FIG. 1 and Table 1. The goal of the depletion study was to evaluate the chemical and functional (staining) changes that occur during sequential staining of numerous slides. In a typical depletion study (Table 1) the hematoxylin, differentiator, bluing agent and eosin were not changed during the entire depletion study while all of the other components (alcohols and xylenes) were rotated or changed at 300 slide intervals. Staining of control tissue specimens on multi-tissue control slides (functional staining) and determination of the pH of the hematoxylin were performed at 150 slide intervals. A standard depletion study incorporated a total of 2,700 slides (90 30-slide racks).
[0047]Other staining runs incorporated an additional buffered reagent of defined composition places between the tap-water rinse and the hematoxylin st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com