Use of Malononitrilamides in Neuropathic Pain
a technology of malononitrilamide and neuropathic pain, which is applied in the field of malonitrilamides, can solve the problems of peripheral neuropathic pain, severe impairment of overall quality of life, and most devastating effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
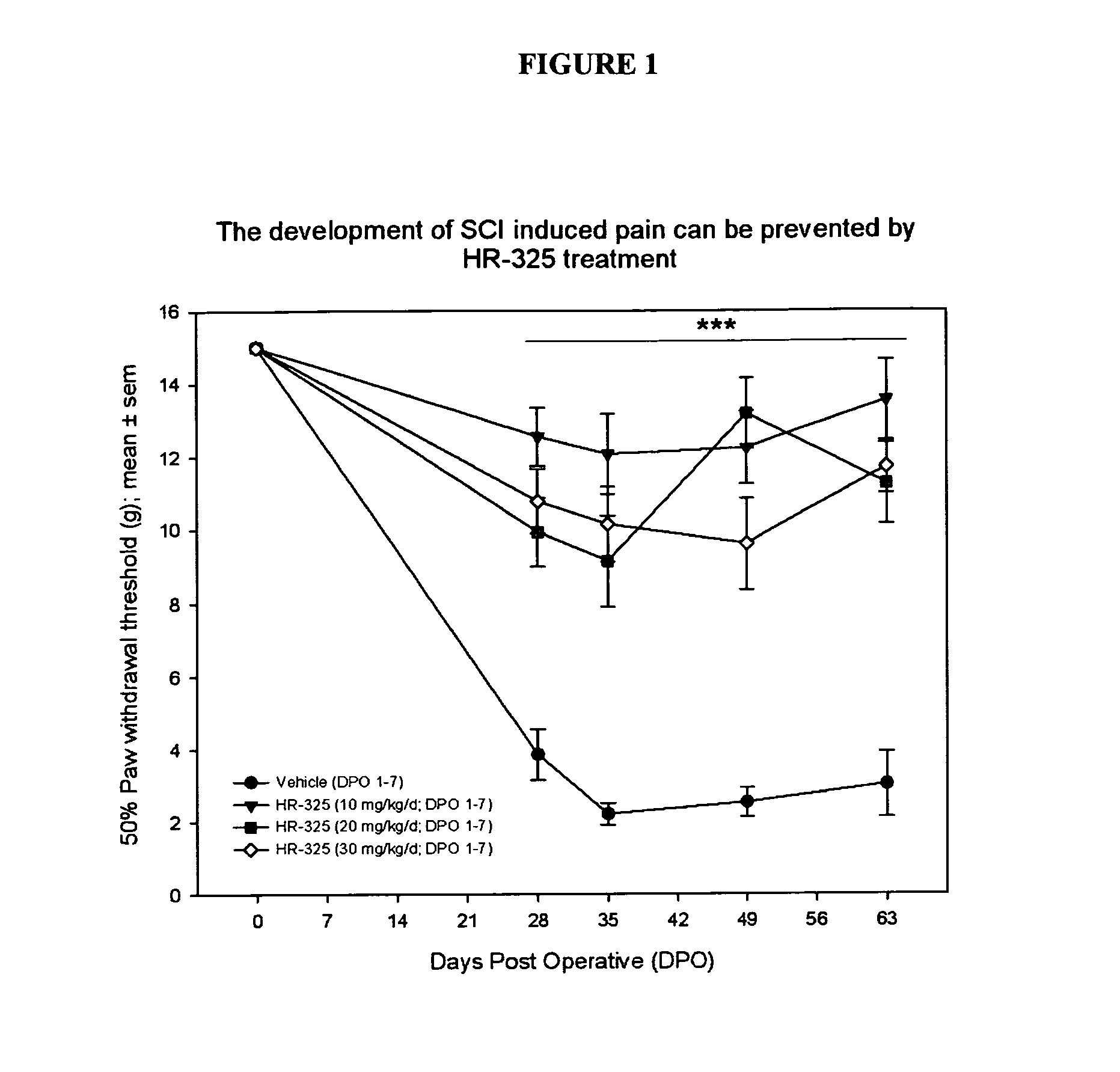
[0138]Laflunimus (HR325) treatment to suppress the development of mechanical allodynia after severe spinal cord contusion injury in the rat.
[0139]Surgical Methods
[0140]Thirteen week-old female Lewis rats (Charles River, Sulzfeld Germany) were housed under a 12:12 h dark / light regime and allowed free access to water and food. After one week of habituation the animals underwent general anesthesia with a mixture of isoflurane and air (induction: 5% isoflurane, maintenance: 2.2% isofluorane). A Th10 laminectomy was performed without rupturing the dura and a severe contusive SCI (25 gcm NYU / MASCIS II impactor) {Gruner, 1992#3} was induced. After suturing muscle and skin, a subcutaneous (s.c.) injection of 5 ml of Ringers Lactate was given. Bladders were emptied manually 2 times a day until spontaneous voiding returned (usually within 1 week). The lesion severity was verified by the impact velocity and contusion depth of the impactor rod. Animals with an impact velocity error >5% were exc...
example 2
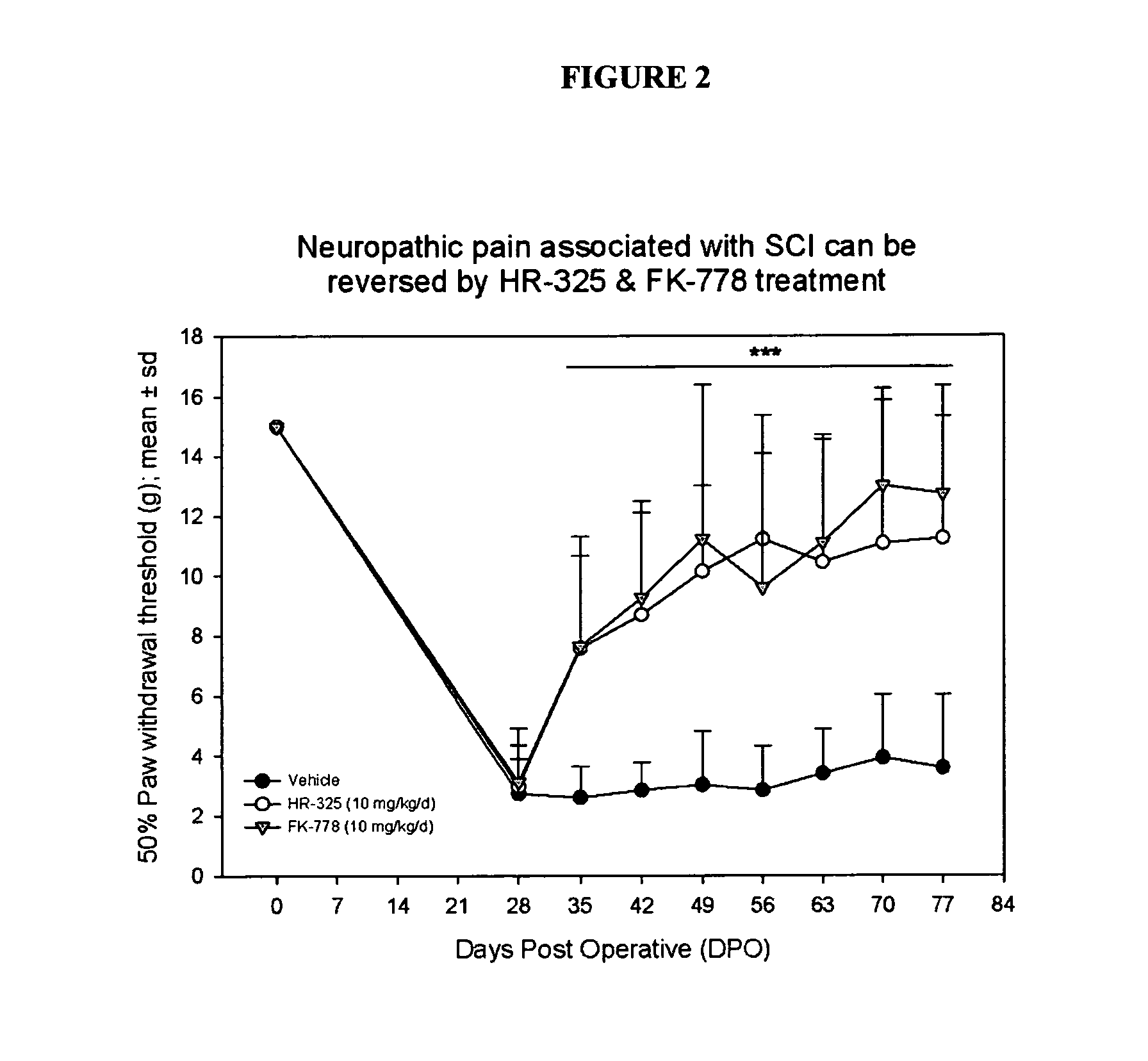
[0146]HR325 and FK778 treatment can both reverse central neuropathic pain induced by severe spinal cord contusion injury in the rat.
[0147]Surgical Methods
[0148]For surgical methods see example 1.
[0149]After injury, individual rats were randomly assigned into a treatment group. The following groups were used:
Group 1: SCI+vehicle (1.5% CMC in sterile water) by gavage for 7 days, from DPO 28 till DPO 35
Group 2: SCI+HR 325 (10 mg / kg / day) in vehicle by gavage for 7 days, from DPO 28 till DPO 35
Group 3: SCI+FK 778 (10 mg / kg / day) in vehicle by gavage for 7 day, from DPO 28 till DPO 35
[0150]Assessment of Mechanical Sensitivity:
[0151]For the assessment of mechanical sensitivity see example 1.
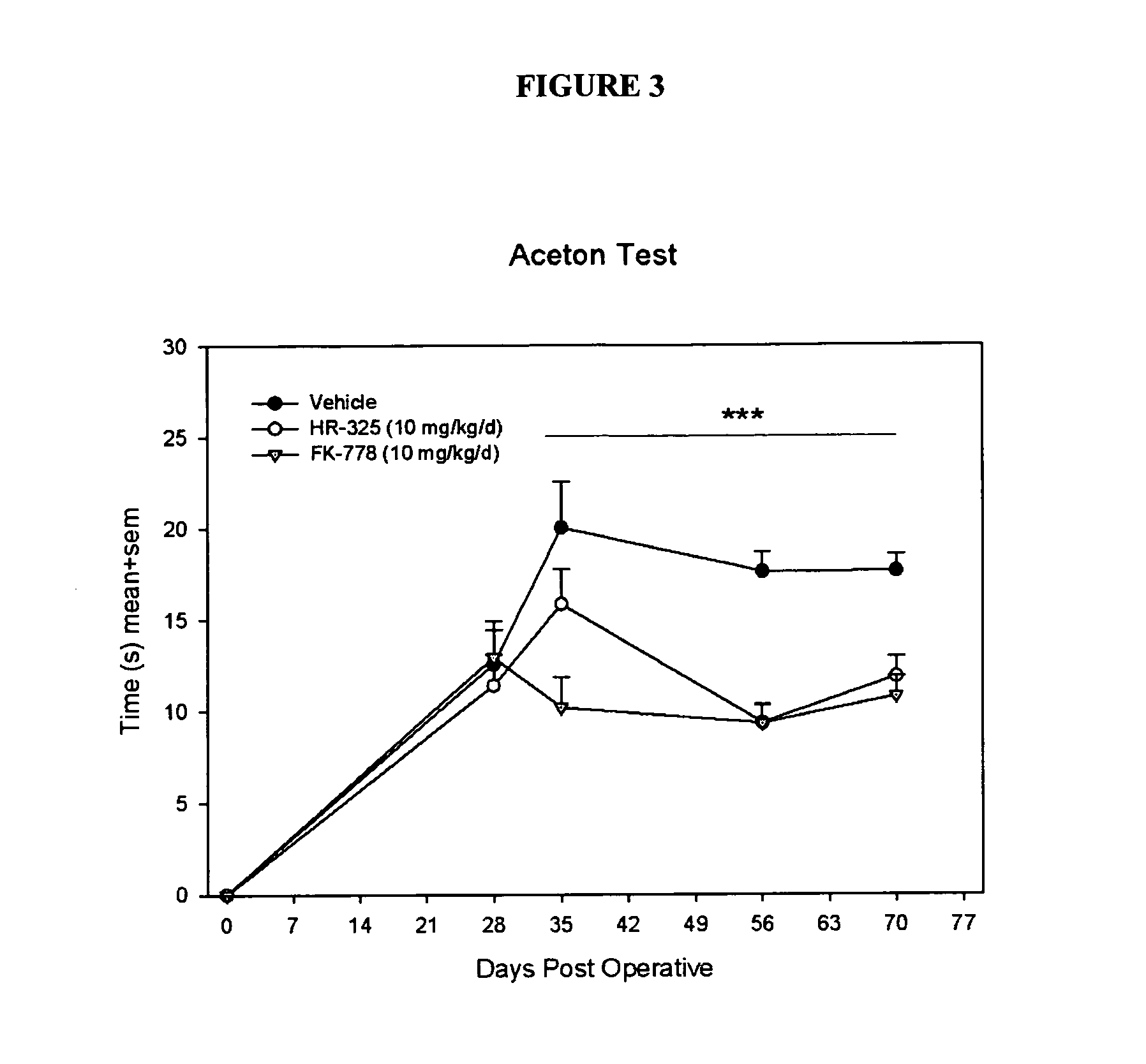
[0152]The Acetone Test:
[0153]A slightly modified method of De la Calle and colleagues (De la Calle et al., 2002) was used for the determination of the reactivity to a cold chemical stimulus. The rat was placed in acrylic cages on top of a wire mesh grid, which allowed access to the paws, and acetone was ...
example 3
[0159]HR325 treatment can reverse chronic central neuropathic pain three months after spinal cord contusion injury.
[0160]Surgical Methods
[0161]For surgical methods see example 1.
[0162]After injury, individual rats were randomly assigned into a treatment group. The following groups were used:
Group 1: SCI+vehicle (1.5% CMC in sterile water) by gavage for 7 days, from DPO 84 till DPO 91
Group 2: SCI+HR 325 (10 mg / kg / day) in vehicle by gavage for 7 days, from DPO 84 till DPO 91
[0163]Assessment of Mechanical Sensitivity:
[0164]For the assessment of mechanical sensitivity see example 1.
[0165]The Acetone Test:
[0166]For the assessment of mechanical sensitivity see example 2.
[0167]Results:
[0168]Mechanical Sensitivity
[0169]The mechanical sensitivity (indicated by the 50% threshold force for paw withdrawals) was determined by the Up-Down method using von Frey filaments. All rats were baseline tested before surgery and tested again on day 28 post surgery, because this is the first time point at w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| paw withdrawal threshold | aaaaa | aaaaa |
| 50% paw withdrawal threshold | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com