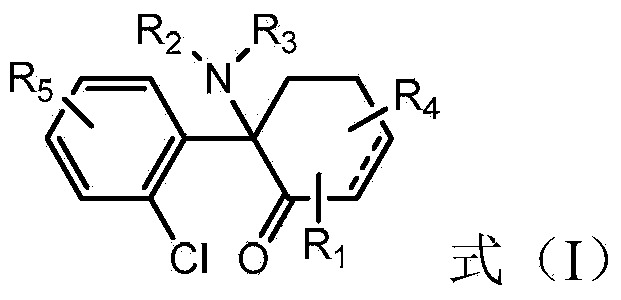
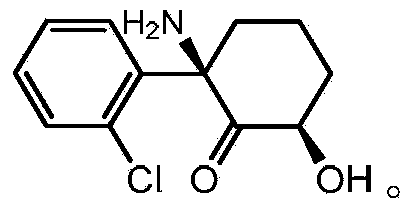
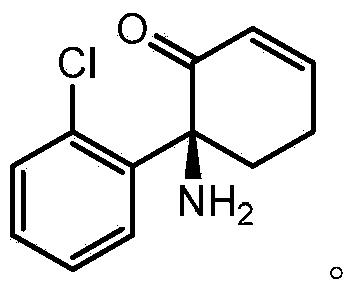
The use of (2r, 6r)-hydroxynorketamine, (s)-dehydronorketamine and other stereoisomeric dehydro and hydroxylated metabolites of (r,s)- ketamine in the treatment of depression and neuropathic pain
A hydroxyl, compound technology for (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydrogenated and hydroxylated metabolites of (R,S)-ketamine Areas of application in the treatment of depression and neuropathic pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Example 1. Synthesis of lysine conjugates of norketamine
[0156]
Embodiment 26
[0157] Synthesis of the ester conjugate of embodiment 2.6-hydroxynorketamine
[0158]
Embodiment 3
[0159] Example 3. Synthesis of (+,-)-(2S,6R / 2R,6S)-6-hydroxynorketamine
[0160]
[0161] Synthesis of (+,-)-(Z+E)-6-bromonorketamine. A 50 mL sample of racemic (+,-)-norketamine (free base) (10.0 g, 35.8 mmol) in glacial acetic acid was treated with bromopyridine (16.4 g, 51.3 mmol). The resulting mixture was heated at 130 °C for 1 h using a microwave. The solvent was removed in vacuo and the crude was dissolved in CHCl 3 , with saturated NaHCO 3 Washed, dried in vacuum (Na 2 SO 4 ) and evaporated, leaving 12.4 g of a crude mixture of diastereoisomers (Z+E, 3:1). Using silica gel chromatography with CH varying in concentration from (99.9 / 0 / 0.1) to (98.9 / 1 / 0.1) 2 Cl 2 / MeOH / Et 3 N eluted to afford the pure isolated isomers, (+,-)-(E)-6-bromonorketamine (1.22 g) (9% yield) and (+,-)-(Z) - 6-bromonorketamine (6.6 g) (49% yield).
[0162] Analytical data for (+,-)-(E)-6-bromonorketamine: 1 H NMR: (300MHz, CDCl 3 ):δ7.60(m,1H),7.20-7.10(m,3H),5.17(dd,1H,J=12.0Hz,J=6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com