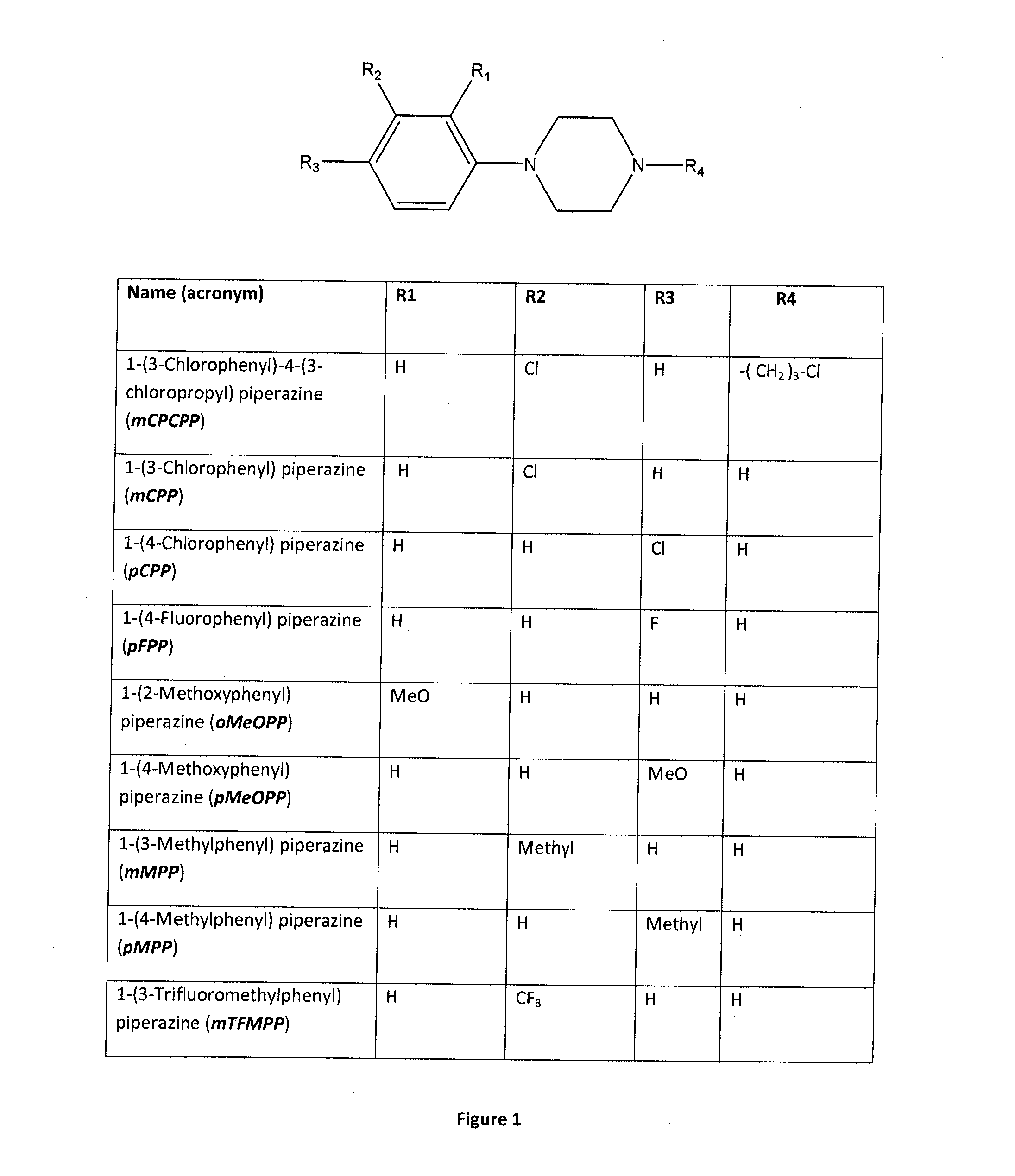
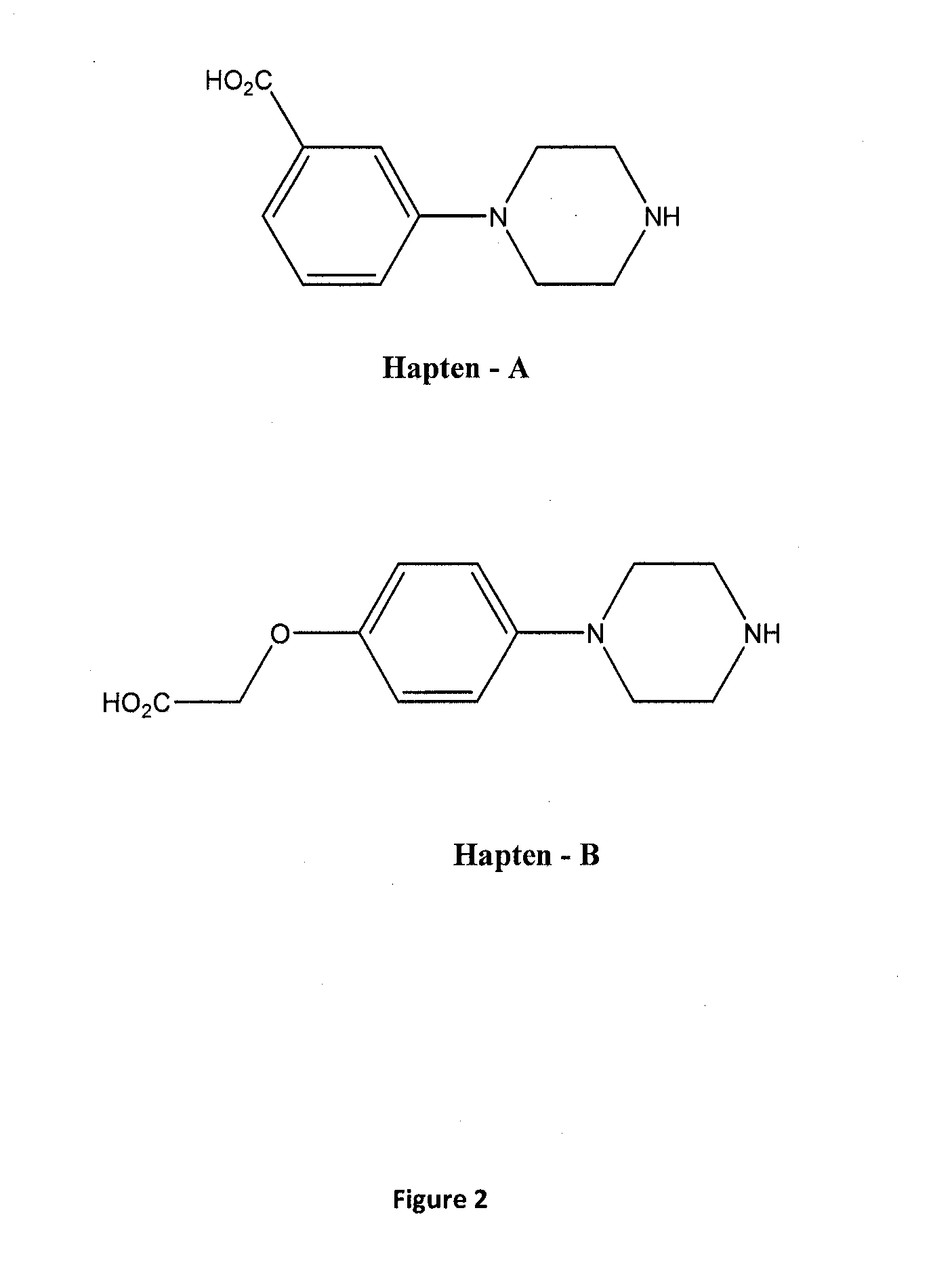
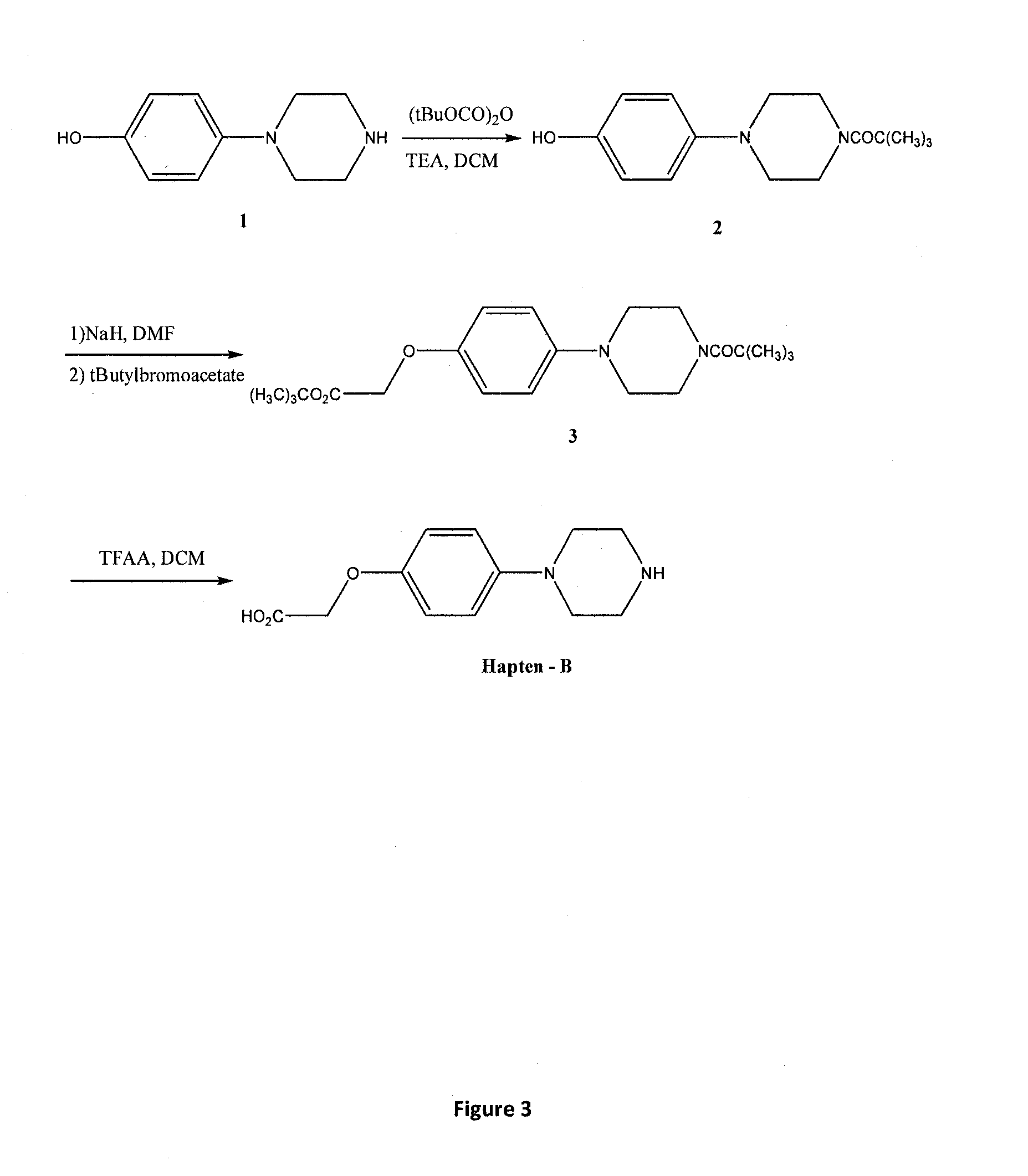
Assay for the Detection of the Phenylpiperazine Family
a technology of phenylpiperazine and detection method, which is applied in the field of piperazine derivatives, can solve the problems of expensive equipment and highly trained staff, and the detection level of this kit is insufficient for testing purposes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Conjugation of 3-(carboxyphenyl)piperazine (Hapten-A) to BSA (to form Immunogen-I)
[0065]To a solution of 3-(carboxyphenyl)piperazine (Hapten-A) (30.9 mg, 0.15 mM) in DMF (1.0 ml) was added N,N-dicyclohexylcarbodiimide (DCC) (33.08 mg, 0.16 mM) and N-hydroxysuccinimide (18.7 mg, 0.16 mM) and the mixture was stirred at room temperature overnight. The dicyclohexylurea formed was removed by filtration and the solution was added dropwise to a solution of BSA (150 mg, 2.3 mmol) in 50 mM sodium bicarbonate solution (pH 8.5) (10 ml). The mixture was then stirred overnight at 4° C. The solution was then dialysed against 50 mM phosphate buffer pH 7.2 (3 changes) for 24 hours at 4° C., and freeze-dried to give Immunogen-I.
[0066]MALDI results showed 14.07 molecules of Hapten-A had been conjugated to one molecule of BSA.
example 2
Conjugation of 3-(carboxyphenyl)piperazine (Hapten-A) to BTG (to form Immunogen-II)
[0067]To a solution of 3-(carboxyphenyl)piperazine (Hapten-A) (41.8 mg, 0.203 mmol) in DMF (1.0 ml) was added N,N-dicyclohexylcarbodiimide (DCC) (46.01 mg, 0.223 mmol) and N-hydroxysuccinimide (25.66 mg, 0.223 mmol) and the mixture was stirred at room temperature overnight. The dicyclohexylurea formed was removed by filtration and the solution was added dropwise to a solution of BTG (150 mg, 2.25 μmol) in 50 mM sodium bicarbonate solution (pH 8.5) (10 ml). The mixture was then stirred overnight at 4° C. The solution was then dialysed against 50 mM phosphate buffer pH 7.2 (3 changes) for 24 hours at 4° C., and freeze-dried to give Immunogen-II.
example 3
Conjugation of 3-(carboxyphenyl)piperazine (Hapten-A) to HRP
[0068]EDC hydrochloride (10 mg) was dissolved in water (0.5 ml) and immediately added to a solution of 3-(carboxyphenyl)piperazine (Hapten-A) (2 mg) in DMF (0.2 ml). After mixing, this solution was added dropwise to a solution of HRP (20 mg) in water (1 ml). Sulfo-NHS (5 mg) was added and the reaction mixture was incubated in the dark at room temperature overnight. Excess hapten was removed with double PD-10 columns (Pharmacia) in series, pre-equilibrated with PBS at pH 7.2. The hapten-A-HRP conjugate was then dialysed overnight against 10 L of PBS at pH 7.2 at 4° C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com