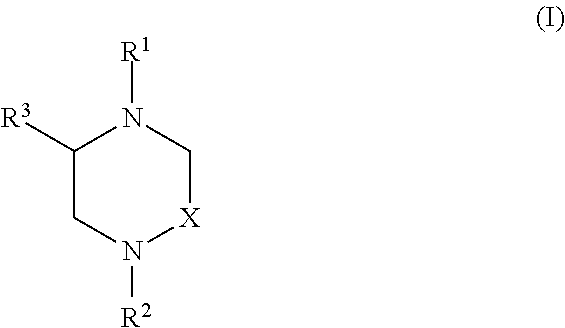
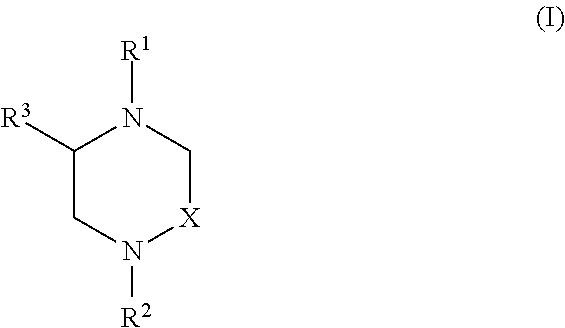
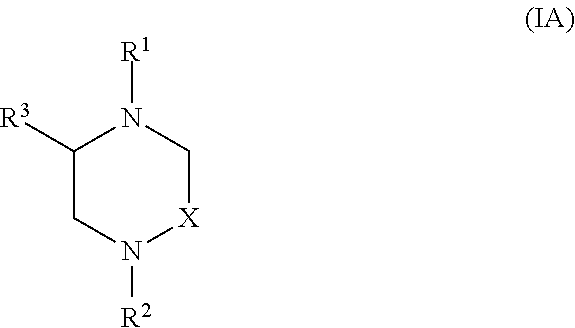
Heterocyclic Compounds and Methods For Their Use
a technology of heterocyclic compounds and at2 receptors, applied in the field of compounds, can solve the problems of neuropathic pain, inflammatory pain, and other types of pain that are difficult to treat, and achieve the effect of treating or preventing a cell proliferative disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Compound 4 (S)-1-(2,2-diphenylacetyl)-4-phenylpiperazine-2-carboxylic acid
[0171]1. Procedure for the Preparation of 4b
[0172]To a stirred solution of compound 4a (100 mg, 0.41 mmol) and PhB(OH)2 (75 mg, 0.61 mmol) in DCM (2 mL) was added Cu(OAc)2 (22 mg, 0.12 mmol) at RT and the mixture was stirred overnight, TLC (MeOH:DCM=1:10) showed most of starting material was consumed. The reaction was repeated on a larger batch of compound 4a (1.0 g, 4.1 mmol) and the reaction mixtures were combined and washed with cold water (20 mL) then brine (10 mL×2), dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by chromatography (EA:PE=1:50) to give 4b (450 mg, 31%) as a colorless oil. LC-MS (Agilent): Rt 3.35 min; m / z calculated for C17H24N2O4 [M+H]+ 321.2, [M+Na]+343.2. found [M+H]+321.1, [M+Na]+343.1.
[0173]2. Procedure for the Preparation of 4c
[0174]To a stirred solution of 4b (0.45 g, 1.4 mmol) in DCM (5 mL) was added CF3COOH (0.96 g, 8.4 mmol) at RT and the mixture ...
example 2
Compound 5 (S)-4-benzyl-1-(2,2-diphenylacetyl)piperazine-2-carboxylic acid
[0179]1. Procedure for the Preparation of Compound 5a
[0180]To a solution of 4a (0.5 g, 2.0 mmol) in DMF (10 mL) at 0° C. was added DIPEA (317.4 mg, 2.45 mmol) and benzyl bromide (359.1 mg, 2.1 mmol) and the mixture was stirred at RT for 40 min, TLC (PE:EA=4:1) showed that the starting material was consumed. Water (30 mL) was added and the mixture was extracted with EA (30 mL). The organic extract was washed with brine, dried over Na2SO4 and concentrated in vacuo to give 5a (650 mg, 97%) as a yellow oil. LC-MS (Agilent): Rt 3.28 min; m / z calculated for C18H26N2O4 [M+H]+ 335.1. found [M+H]+ 355.1.
[0181]2. Procedure for the Preparation of Compound 5b
[0182]To a solution of 5a (650 mg, 1.95 mmol) in DCM (8 mL) was added TFA (1.34 g, 11.7 mmol) and the mixture was stirred at RT overnight, TLC (PE:EA=4:1) showed that the starting material was consumed. The solvent was removed in vacuo, water (15 mL) and Et2O (15 mL) ...
example 3
Compound 6 (S)-4-(1,5-diphenyl-1H-pyrazol-3-yl)-1-(2,2-diphenylacetyl)-piperazine-2-carboxylic acid
[0187]1. Procedure for the Preparation of 6b
[0188]A mixture of the 4a (2.00 g, 13.8 mmol) and 6a (9.34 g, 41.6 mmol) (prepared according to the procedure in Tetrahedron, 2010, 66, 2843) in toluene (40 mL) was heated at 130° C. in a sealed tube for 3 hours, TLC (DCM:MeOH=20:1) showed the starting material was consumed. The solvent was removed in vacuo and the residue was purified by Al2O3 column (PE:EA=10:1 to 4:1) to give 6b (600 mg, 16%) as a thick yellow oil. LC-MS (Agilent): Rt 3.27 min; m / z calculated for C21H28N2O5S [M+H]+ 421.2, [M+Na]+443.2. found [M+H]+421.2, [M+Na]+443.2.
[0189]2. Procedure for the Preparation of Compound 6c
[0190]A mixture of 6b (600 mg, 1.42 mmol), DABCO (192 mg, 1.71 mmol) and PhNHNH2 (185 mg, 1.71 mmol) in t-BuOH (30 mL) was heated at reflux overnight, TLC (PE:EtOAc=2:1) showed most of the starting material was consumed. The mixture was cooled to RT and conc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com