Anti-mcam antibodies and associated methods of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
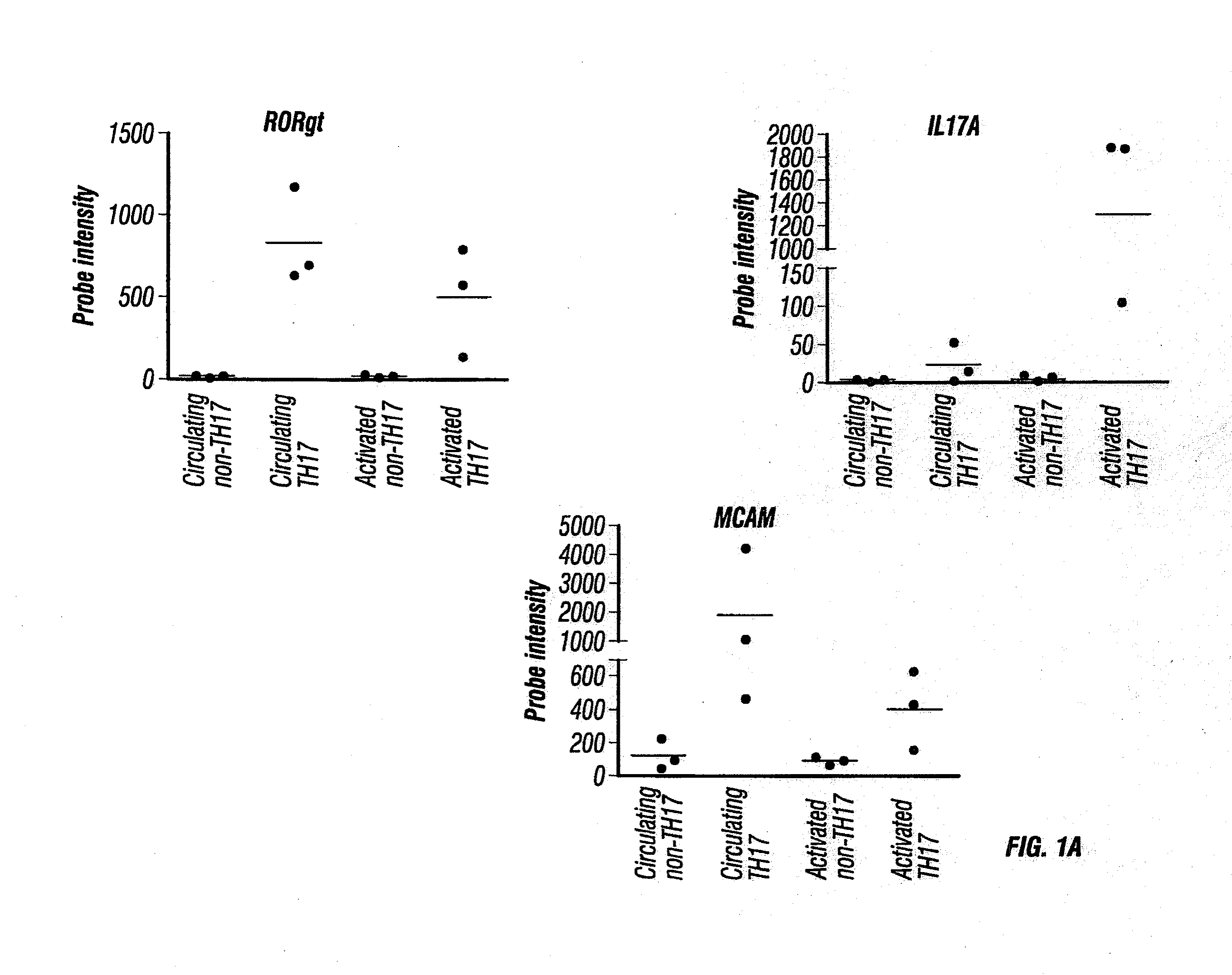
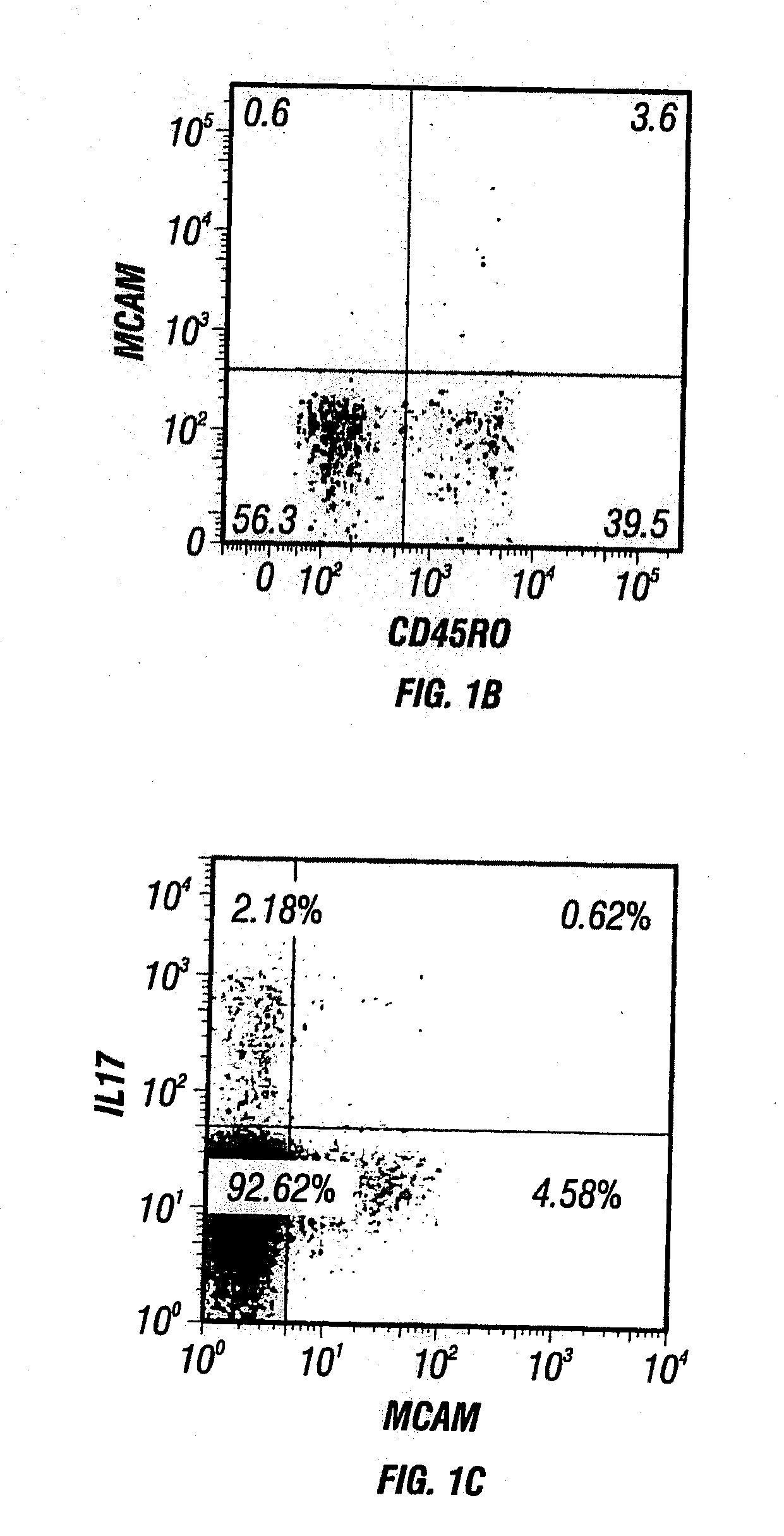
MCAM is a Gene Up-Regulated in IL-17-Producing Human CD4+ T Cells
[0442]To identify novel targetable molecules associated with TH17 cell infiltration of the CNS, human CD4+ T cells from three healthy donors were enriched by magnetic negative selection as described in Materials and Methods above. After the enriched human CD4+ T cells were stained for surface expression of CD161 and CCR6, cells were FACS sorted into two populations: CCR6− / CD161− (representing circulating non-TH17 cells) and CCR6+ / CD161+(representing circulating TH17 cells) as described in Materials and Methods above. RNA was isolated immediately from half of the cells in each population as described in Materials and Methods above. The other half was put into culture with plate-bound anti-CD3 and soluble anti-CD28, without exogenous cytokines, for four days to obtain activated non-TH17 cells and activated TH17 cells, respectively. RNA was similarly isolated from these two types of activated cells. RNA was subject to mic...
example 2
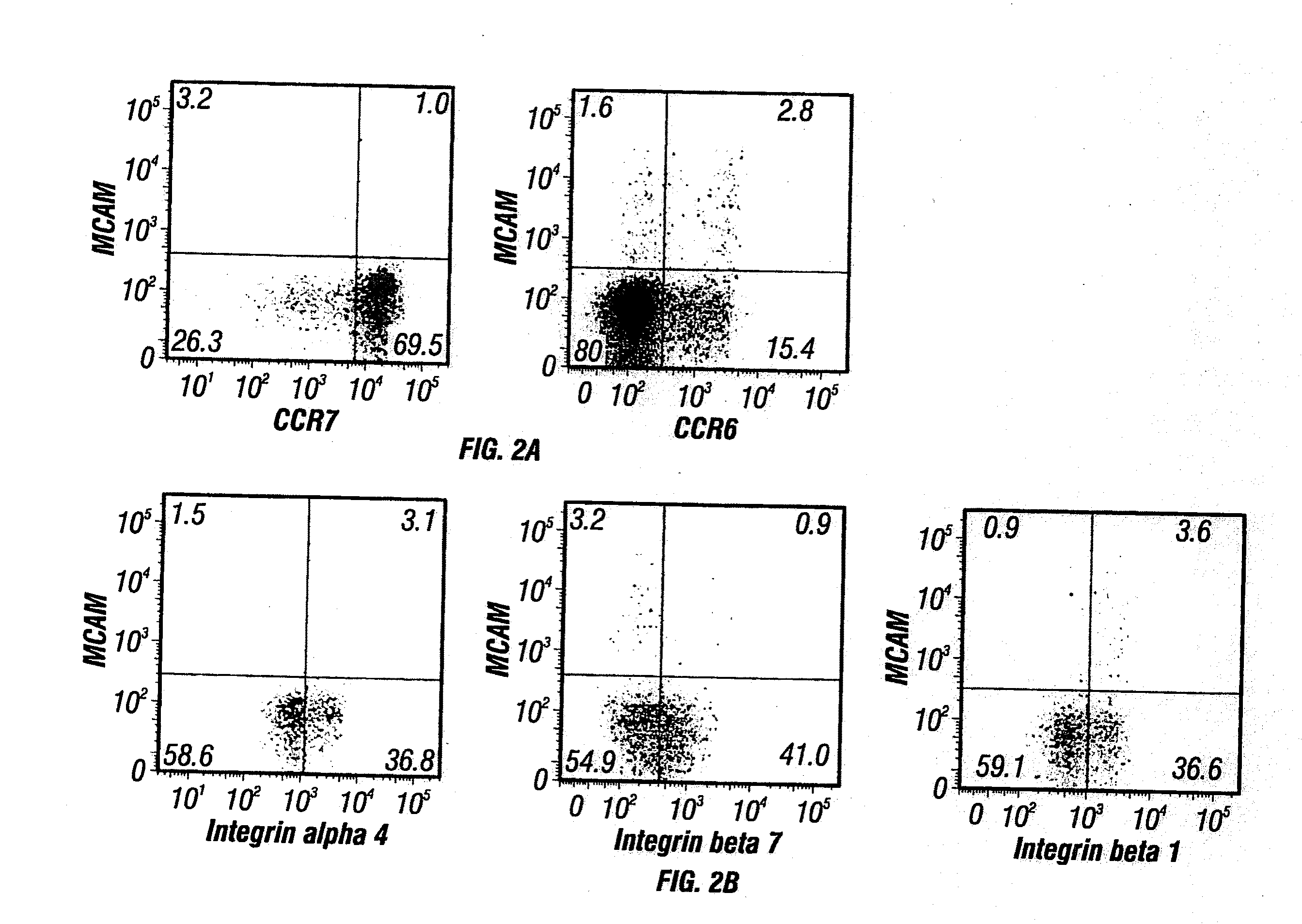
MCAM Expressing T Cells are Effector Memory T Cells Having a Unique Integrin Expression Profile
[0446]The CD45RO+ memory population of human CD4 T cells can be segregated into (1) effector memory cells with tissue tropism, and (2) central memory cells with lymphoid tissue homing based upon expression of CCR7. See, e.g., Sallusto et al., Nature 401: 708-712 (1999).
[0447]To determine which subpopulation includes the MCAM expressing T cells, MCAM expression in T cells was further characterized by staining peripheral human T cells with various markers (CCR6, CCR7, integrin subunits alpha 4, beta 1, and beta 7) as described in Materials and Methods above. MCAM expressing CD4+ T cells were largely CCR7 negative, indicating that most are effector memory T cells, and would be more likely to home to tissues (FIG. 2A). The TH17 enrichment protocol suggested that MCAM expressing T cells obtained would be disproportionately CCR6+. As shown in FIG. 2A, about 64% of MCAM+ cells (2.8% / (2.8%+1.6%)) ...
example 3
MCAM Expressing T Cells are Expanded by IL1β and Produce the Majority of Both IL-17 and IL-22 Under TH17 Conditions
[0449]MCAM expressing CD4+ T cells, at only 3-5% of cells, is a small minority of the T cell population. It is of interest to determine the conditions under which this population expands and exerts TH17 effector function. For this, human CD4+ / CD45RO+ T cells were purified as described in Materials and Methods above and stimulated in vitro with anti-CD3 and anti-CD28 in the presence of a number of cytokine conditions (TGFβ, IL-12, IL-1β, IL-23, and various combinations), and the percentage of MCAM expressing cells, as well as IL-17 expressing cells, was determined by flow cytometry (FIG. 3A). MCAM expression expanded upon stimulation with IL-1β alone (16.4% in the absence of IL-1β vs. 38.1% in the presence of IL-1β, FIG. 3B). Furthermore, while TGFβ alone did not expand the MCAM positive population greatly, it functioned synergistically with IL-1β, as the combination of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com