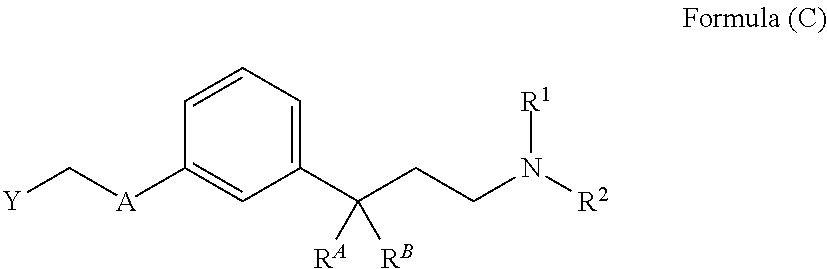
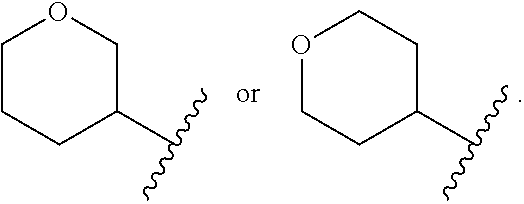
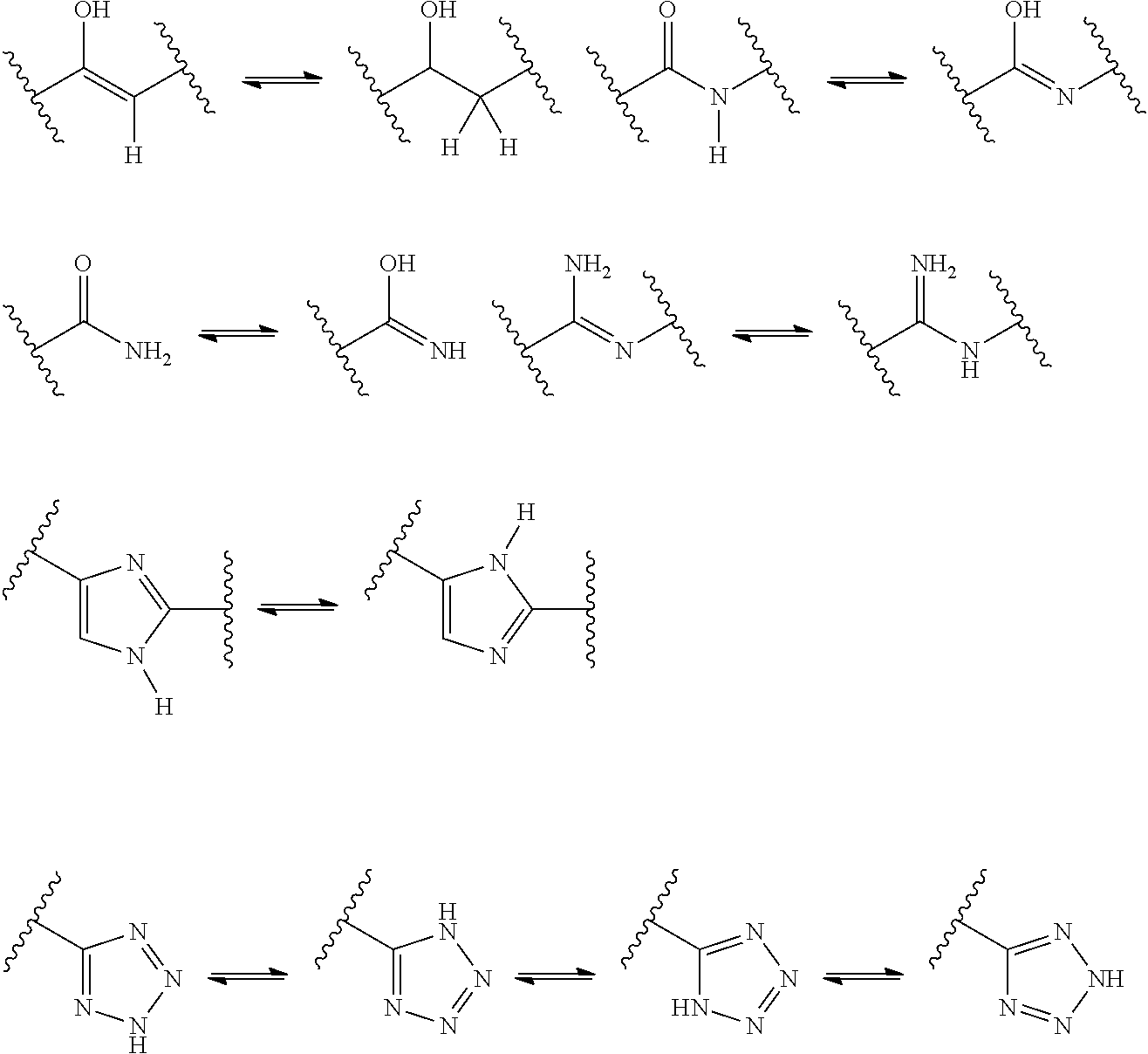
Substituted 3-phenylpropylamine derivatives for the treatment of ophthalmic diseases and disorders
a technology of ophthalmic diseases and derivatives, which is applied in the field of substituting 3phenylpropylamine derivatives for the treatment of ophthalmic diseases and disorders, can solve the problems of generating photochemical damage to the retina
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 6
Preparation of (R)-3-amino-1-(3-(pyridin-2-ylmethoxy)phenyl)propan-1-ol
[0443]
[0444](R)-3-amino-1-(3-(pyridin-2-ylmethoxy)phenyl)propan-1-ol was prepared following the method used in Example 4:
[0445]Step 1: Reaction between 2-(bromomethyl)pyridine and phenol (7, Intermediate I) following the method used in Example 4 gave after purification by flash chromatography (40% EtOAc:hexane) (R)-tert-butyl (3-hydroxy-3-(3-(pyridin-2-ylmethoxy)phenyl)propyl)carbamate as a pale yellow solid. Yield (0.26 g, 88%); 1H NMR (400 MHz, CDCl3): δ 8.59 (d, J=4.8 Hz, 1H), 7.71 (t, J=7.6, 1.6 Hz, 1H), 7.52 (d, J=7.6 Hz, 1H), 7.27-7.20 (m, 2H), 7.03 (s, 1H), 6.96 (d, J=10.4 Hz, 1H), 6.87 (d, J=5.6 Hz, 1H), 5.21 (s, 2H), 4.87 (bs, 1H), 4.73-4.70 (m, 1H), 3.50-3.48 (m, 1H), 3.21-3.11 (m, 1H), 1.84-1.61 (m, 2H), 1.45 (s, 9H); MS: m / z 359.26 [M+H]+.
[0446]Step 2: Deprotection of (R)-tert-butyl (3-hydroxy-3-(3-(pyridin-2-ylmethoxy)phenyl)propyl)carbamate following the method used in Example 4 gave after purificat...
example 7
Preparation of (1R)-3-amino-1-(3-((tetrahydro-2H-thiopyran-3-yl)methoxy)phenyl)propan-1-ol
[0447]
[0448](1R)-3-Amino-1-(3-((tetrahydro-2H-thiopyran-3-yl)methoxy)phenyl)propan-1-ol was prepared following the method below.
[0449]Step 1: K2CO3 (0.72 g, 5.24 mmol) and phenol (7, Intermediate I) (0.3 g, 1.05 mmol) were added to a solution of (tetrahydro-2H-thiopyran-3-yl)methyl 4-methylbenzenesulfonate (0.29 g, 1.10 mmol) in DMF (10 mL). The reaction mixture was heated at 100° C. under reflux overnight and then cooled. The reaction mixture was extracted with EtOAc, organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. Purification by flash chromatography (EtOAc:hexane 10-40% gradient) gave tert-butyl ((3R)-3-hydroxy-3-(3-((tetrahydro-2H-thiopyran-3-yl)methoxy)phenyl)propyl)carbamate as a light green solid. Yield (0.21 g, 52%); 1H NMR (400 MHz, CDCl3): δ 7.24 (t, J=8.0 Hz, 1H), 6.91 (d, J=8.0 Hz, 2H), 6.78 (d, 1H), 4.87 (bs, 1H), 4.71 (bs, 1H), 3.84-3.80 (m, ...
example 8
Preparation of (R)-3-amino-1-(3-((tetrahydro-2H-thiopyran-4-yl)methoxy)phenyl)propan-1-ol
[0451]
[0452](R)-3-Amino-1-(3-((tetrahydro-2H-thiopyran-4-yl)methoxy)phenyl)propan-1-ol was prepared following the method used in Example 7:
[0453]Step 1: Pyridine (7.6 mL, 98.4 mmol) was added at 0° C. to a solution of (tetrahydro-2H-thiopyran-4-yl)methanol (1.3 g, 9.84 mmol) in CH2Cl2 (20 mL) followed by the addition of TsCl (2.0 g, 10.83 mmol). The reaction mixture was stirred at ambient temperature for 18 h, diluted with H2O (200 mL) and extracted with CH2Cl2 (200 mL). Drying over anhydrous Na2SO4 followed by concentration in vacuo gave (tetrahydro-2H-thiopyran-4-yl)methyl 4-methylbenzenesulfonate as an off white solid. The crude was carried forward to the next step without additional purification. Yield (0.75 g, 30%).
[0454]Step 2: Reaction between (tetrahydro-2H-thiopyran-4-yl)methyl 4-methylbenzenesulfonate and phenol (7, Intermediate I) following the method used in Example 7 gave after puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com