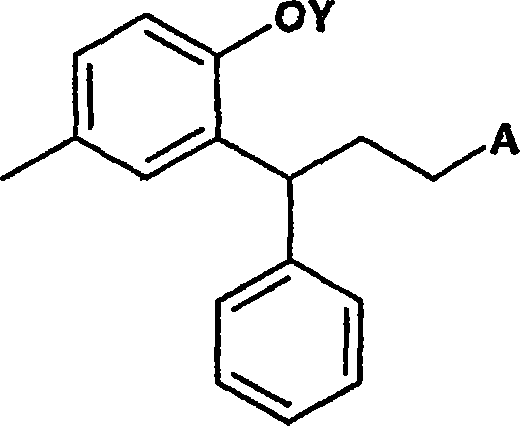
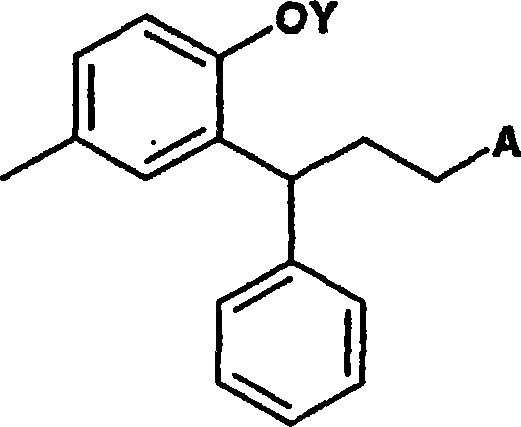
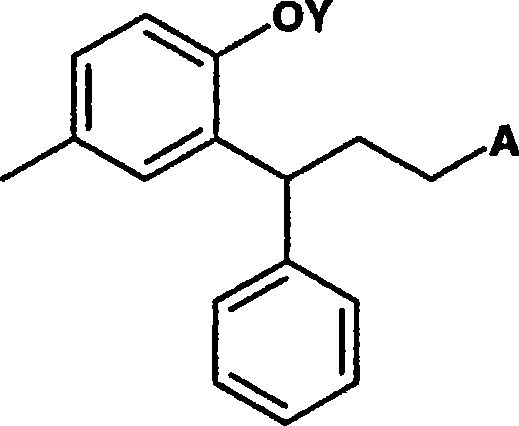
Process for preparation of 3-(2-hydroxy-5-methylphenyl)-n,n-diisopropyl-3phenylpropylamine
A technology of methylphenyl and diisopropyl, applied in the field of organic chemistry, can solve problems such as short time and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] The method of the present invention is described with flow chart, and it comprises the following steps:
[0045] a) reducing the lactone ring of the optionally substituted compound of formula I to open the lactone ring to obtain a compound 2-hydroxyphenylpropanol derivative represented by formula II, which may be optionally substituted, preferably 3, 4-Dihydro-6-methyl-4-phenyl-2-benzopyran-2-one reduces the lactone ring opening to give 3-(2-hydroxy-5-methylphenyl)-3- Phenylpropanol;
[0046] b) Conversion of the two hydroxyl groups of the compound of formula II using the same reagent to form a di-O-substituted derivative by treatment with a reagent that simultaneously activates the alcohol moiety and protects the phenol moiety. Said transformation is characterized in that -O- substituents on the propyl chain react more readily with diisopropylamine than -O-substituents on the aromatic rings to give optionally substituted compounds of formula III;
[0047] c) using ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com