Polycarbonate resin, and polycarbonate resin composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
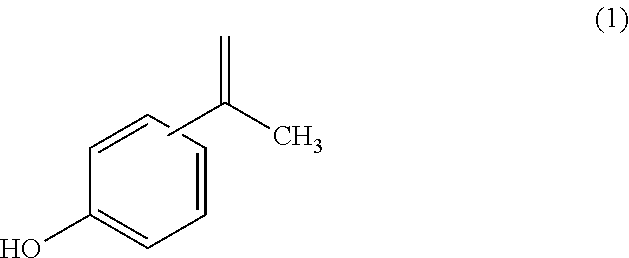
[0073]An obtained bisphenol A (initial IPP concentration: 5 ppm by mass, initial 2,4-isomer concentration: 102 ppm by mass) was heated under the following conditions. That is, 10 g of the bisphenol A was loaded into a colorimetric tube having a diameter of 30 mm to be used in the measurement of an APHA or the like, and was heated in an electric furnace at 175° C. After the heating for 1 hour, the colorimetric tube was removed from the electric furnace and cooled. After that, the solidified bisphenol A was removed and subjected to impurity analysis. The bisphenol A was analyzed for the concentration of isopropenylphenol (hereinafter sometimes abbreviated as “IPP”) and the concentration of the 2,4-isomer by high performance liquid chromatography. A high performance liquid chromatograph (manufactured by Waters, model: 2695, column: manufactured by GL Sciences Inc., Inertsil (trademark) ODS-3V) was used in the analysis including the analysis of the initial concentration. The mobile phas...
example 2
[0082]A bisphenol A having an initial IPP concentration of 6 ppm by mass and an initial 2,4-isomer concentration of 204 ppm by mass was subjected to a heating test in the same manner as in Example 1. Its IPP concentration and 2,4-isomer concentration after the heating test were 32 ppm by mass and 206 ppm by mass, respectively. The results are shown in Table 1-1.
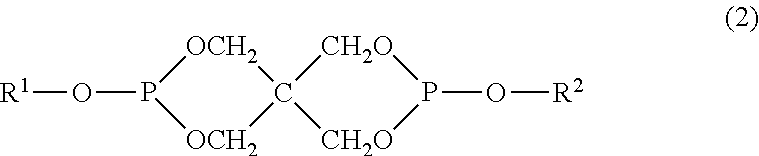
[0083]100 Parts by mass of (A) a polycarbonate resin produced by using a bisphenol A of the same lot as that of the bisphenol A as a raw material in the same manner as in Example 1 was dry-blended with (B-1) the phosphorus-based antioxidant and (C) the polyorganosiloxane having a functional group at ratios shown in Table 2-1, and then a polycarbonate resin composition was prepared in the same manner as in Example 1. Molded bodies were produced from the composition, and their YI values were measured. The results are shown in Table 2-1.
example 3
[0084]A bisphenol A having an initial IPP concentration of 4 ppm by mass and an initial 2,4-isomer concentration of 56 ppm by mass was subjected to a heating test in the same manner as in Example 1. Its IPP concentration and 2,4-isomer concentration after the heating test were 39 ppm by mass and 56 ppm by mass, respectively. The results are shown in Table 1-1.
[0085]100 Parts by mass of (A) a polycarbonate resin produced by using a bisphenol A of the same lot as that of the bisphenol A as a raw material in the same manner as in Example 1 was dry-blended with (B-1) the phosphorus-based antioxidant and (C) the polyorganosiloxane having a functional group at ratios shown in Table 2-1, and then a polycarbonate resin composition was prepared in the same manner as in Example 1. Molded bodies were produced from the composition, and their YI values were measured. The results are shown in Table 2-1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com