Combined enteropathogen recombinant construct
a technology of enteropathogens and recombinant constructs, which is applied in the field of recombinant constructs, can solve the problems of no fda-licensed vaccine for either pathogen, serious health threat to western travelers and young children, and etec is the most common cause of travelers' diarrhea. , to achieve the effect of reducing amino acid sequence length, avoiding undesirable associations, and reducing protease cleavag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Conjugation of ETEC Polypeptides to C. jejuni Capsule Polysaccharide (CPS)
[0036]In a preferred embodiment, ETEC recombinant polypeptides or polypeptide constructs are conjugated to C. jejuni CPS. The CPS can be derived from a number of C. jejuni strains. In the embodiment, any CPS of any C. jejuni strain is envisioned to be conjugated to ETEC recombinant polypeptide constructs. Alternatively, Shigella LPS can be conjugated to ETEC recombinant polypeptide constructs.
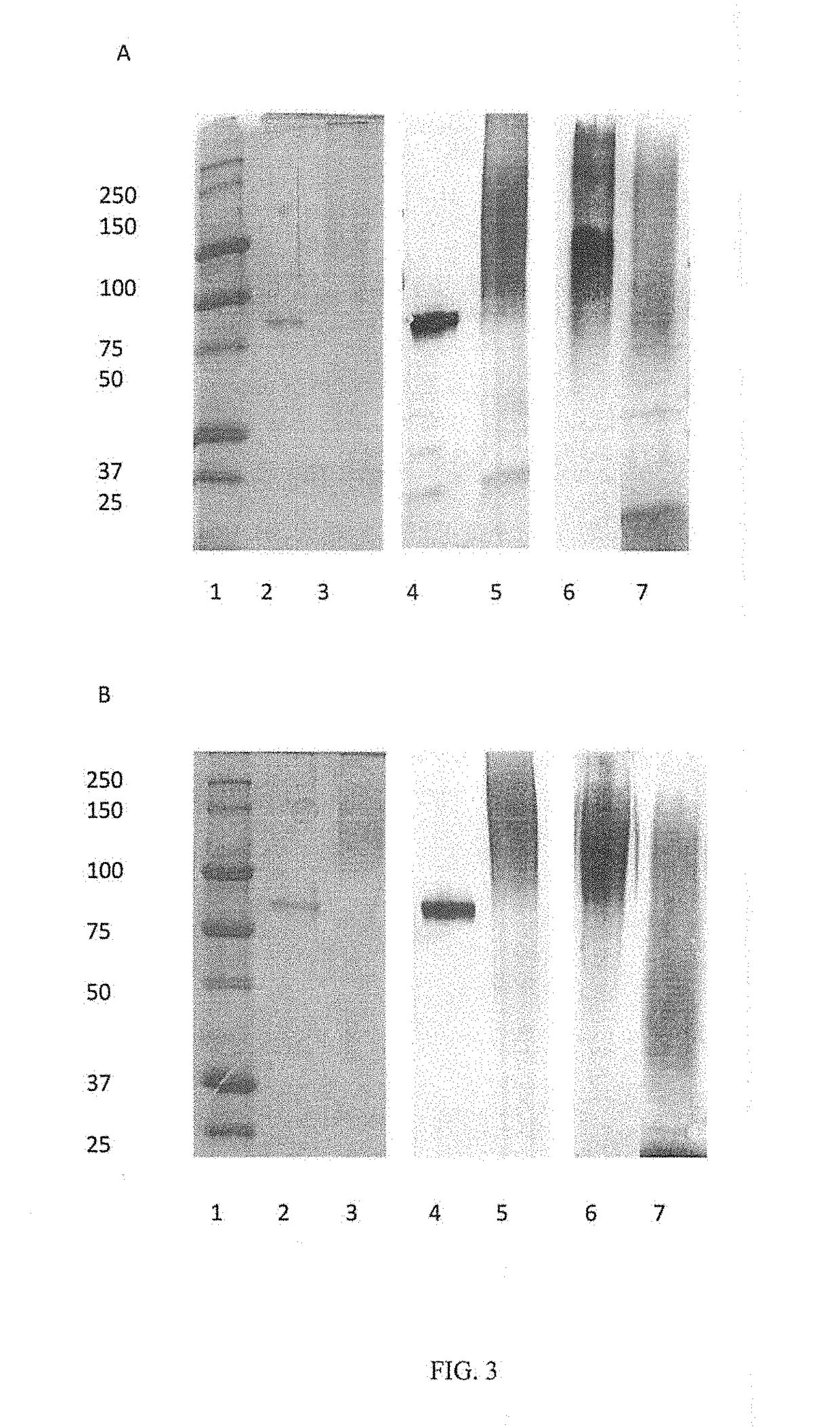
[0037]The overall method of conjugating includes oxidizing C. jejuni CPS, for example, with NaIO4 in sodium acetate (pH 4.0). Oxidized CPSs were desalted with a 5 kDa cutoff membrane by stirred ultrafiltration, which is subsequently lypholized. ETEC proteins are then added. The stoichiometery protein to CPS can vary, however, a typical ratio is 1:2 protein to CPS by mass. The concentration of components can be by any method. However, for example, polysaccharide concentration was determined by antrhone assay and protein co...
example 2
Anti-Class 5 ETEC, CS3 or CS6 Constructs
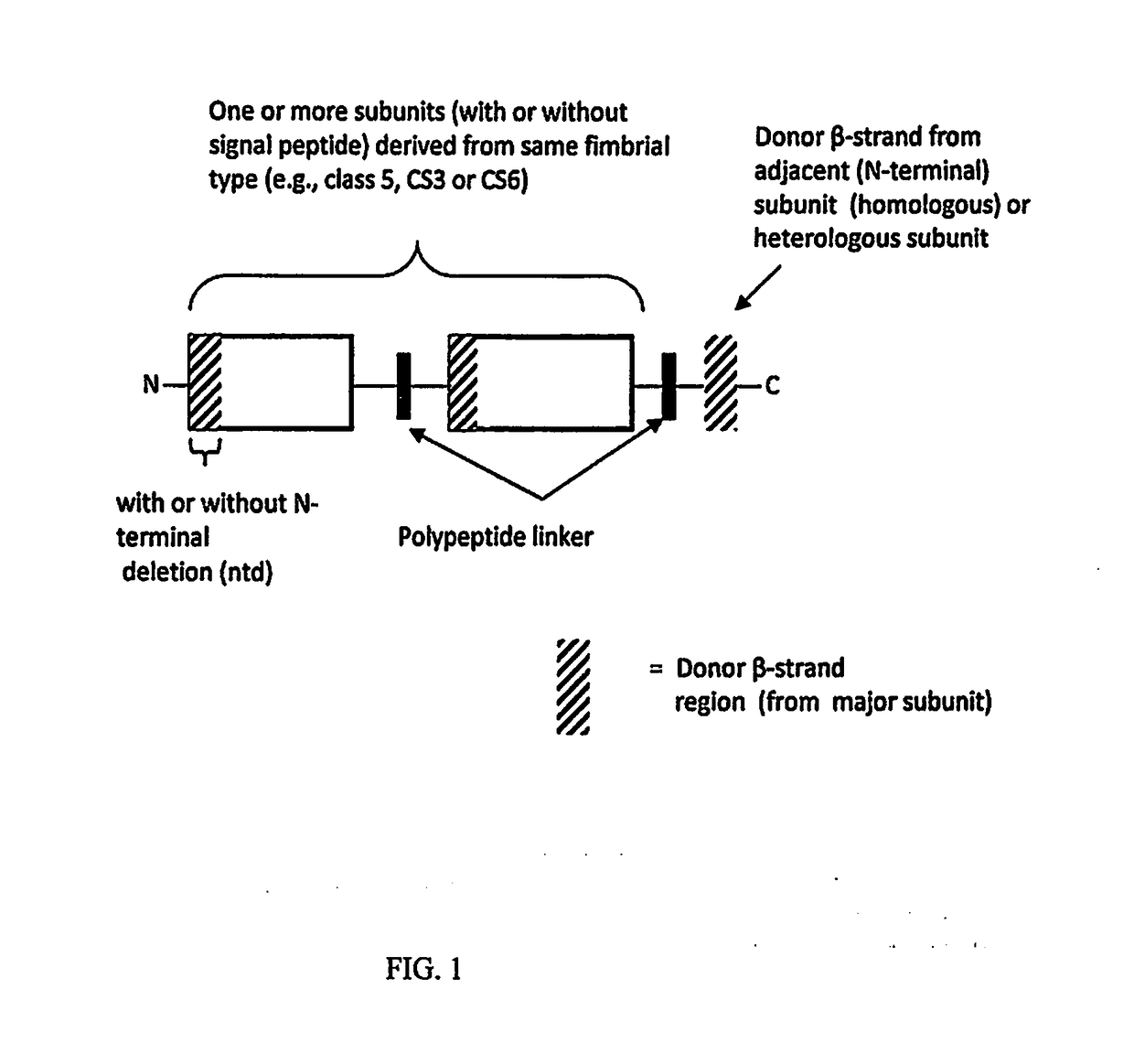
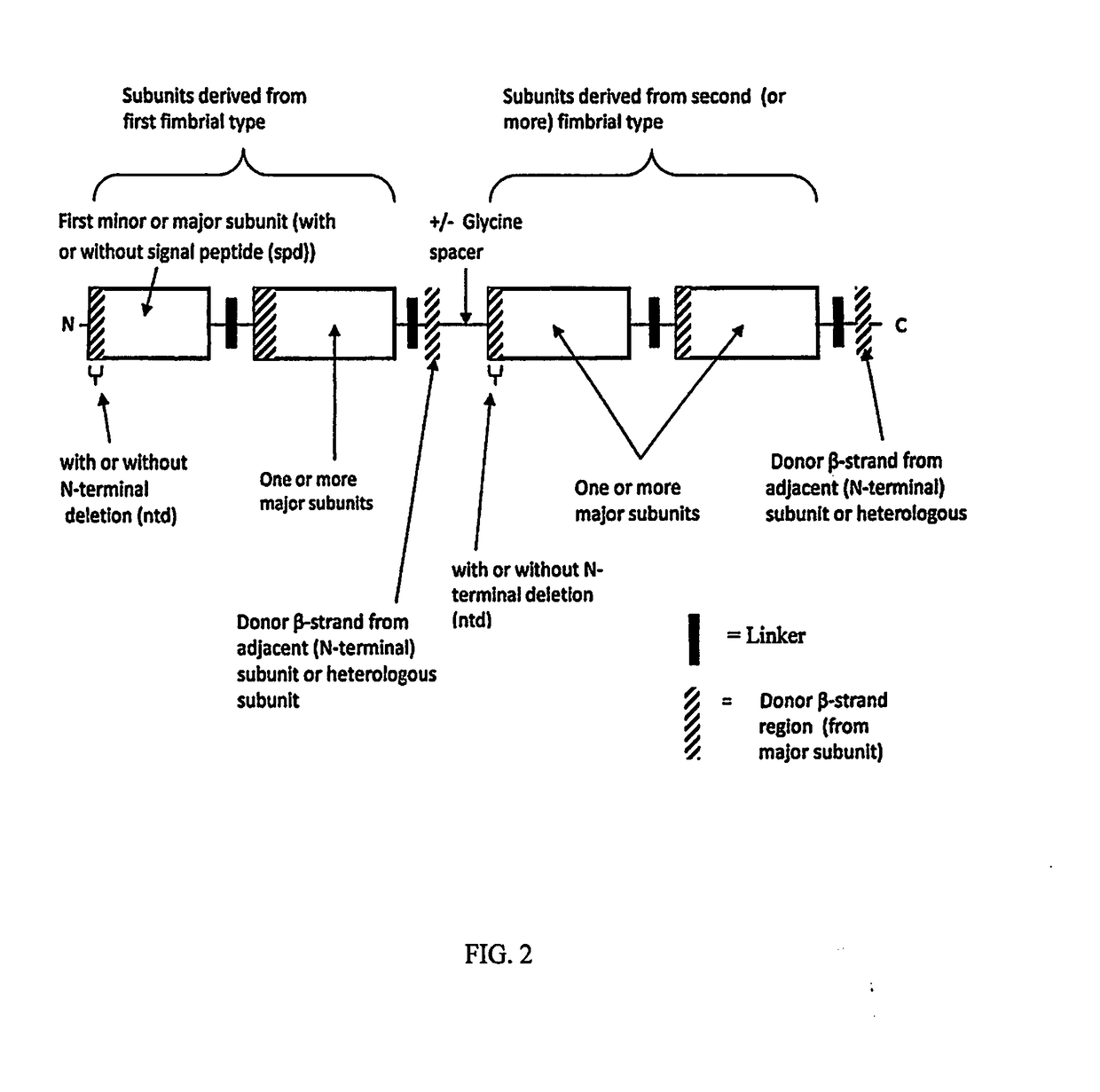
[0047]Anti-ETEC constructs that are contemplated to be conjugated to C. jejuni polysaccharide comprise the structures as illustrated in FIG. 1 and FIG. 2. FIG. 1 illustrates the basic recombinant construct design. As diagrammed in FIG. 1 the construct design comprises one, or more ETEC major or minor fimbrial subunits or fragments of major fimbrial subunits, containing the donor strand, derived from the same ETEC fimbrial type, which are connected, via polypeptide linkers and stabilized by donor strand complementation. The construct can contain a deletion of the N-terminal region of the N-terminal subunit. This feature prevents undesirable associations with other monomers or multimers. The C-terminal subunit is connected to and stabilized by a donor β strand, connected to the subunit via a polypeptide linker, wherein the donor β strand is either derived from the adjacent subunit (i.e., homologous) or from a different subunit of the same fimbri...
example 3
C. jejuni Capsule Polysaccharides
[0099]Recent development of a molecular CPS typing system re-enforced the strong correlation between CPS and Penner types (Poly, et al., J. Clin. Microbiol. 49: 1750 (2011)). Both Penner serotyping and molecular CPS typing have revealed the predominance of a handful of CPS types worldwide. Also, despite over 60 Penner serotypes having been identified, most Campylobacter diarrheal disease is caused by C. jejuni expressing only a limited number of serotypes. Therefore, only selected strains of C. jejuni, predicated on epidemiological studies, provides suitable candidate strains for development of vaccine compositions. However, despite the importance of this organism to human disease, there are no licensed vaccines against C. jejuni.
[0100]C. jejuni capsule polysaccharide (CPS) was extracted from C. jejuni strains selected based on their association with diarrheal disease. CPS from bacteria was extracted by hot water-phenol extraction for 2 h at 70° C. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com