Recombinant polypeptide construct comprising multiple enterotoxigenic Escherichia coli fimbrial subunits
a technology of escherichia coli and polypeptides, which is applied in the direction of peptides, antibacterial agents, antibacterial ingredients, etc., can solve the problems of limited serologic cross-reactivity of native fimbriae, and achieve the effects of reducing amino acid sequence length, avoiding undesirable associations, and reducing protease cleavag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Recombinant anti-ETEC Construct
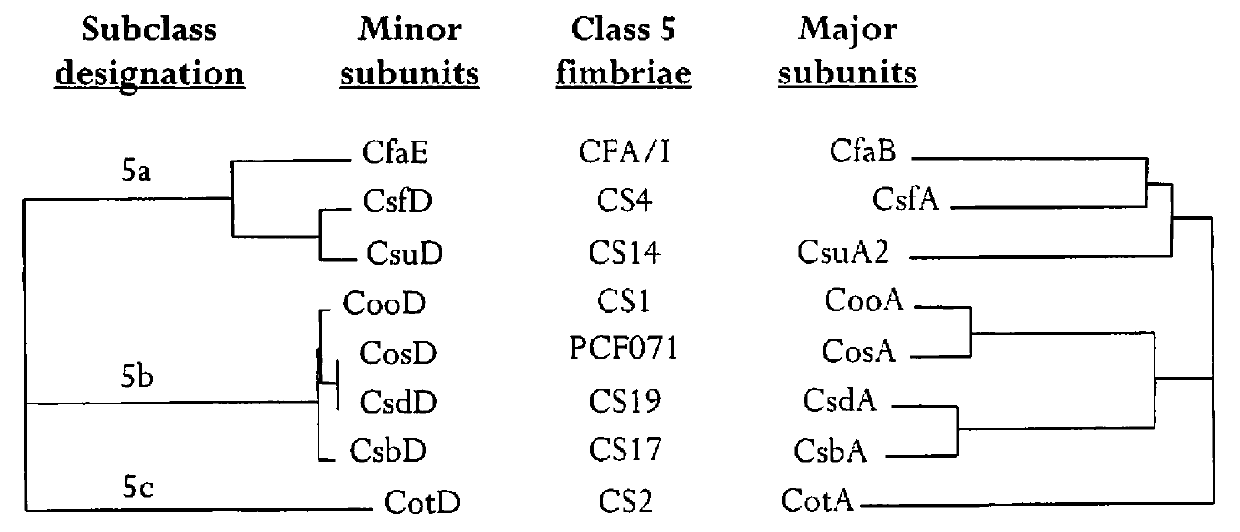
[0053]Immunity to ETEC adhesin is important in reducing colonization of ETEC bacteria. However, the minor subunits (i.e., adhesin), the contact site of ETEC bacteria to the intestimal lumen, of ETEC Class 5 fimbriae are stoichiometrically represented in very low numbers relative to the major subunit. Therefore, in immunogenic compositions, it is important to enhance the immune recognition of the minor subunit over that not normally found in natural fimbriae.
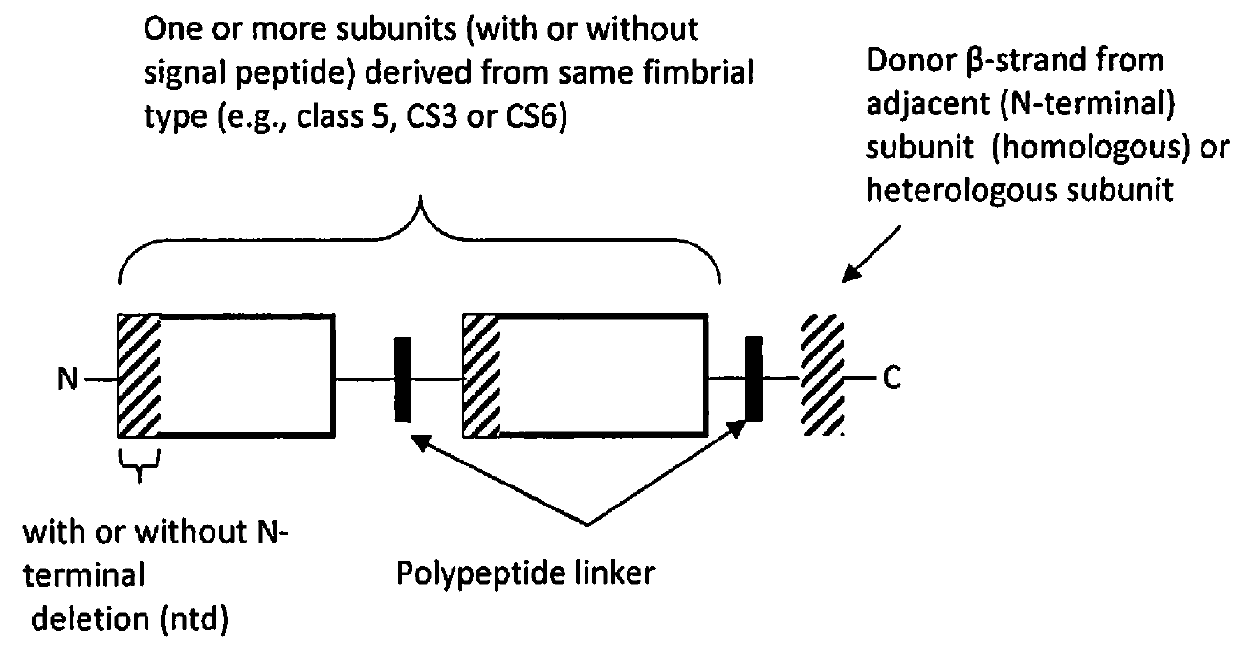
[0054]Since fimbrial subunits, such as CfaE, are relatively susceptible to proteolytic degradation outside of the fimbrial structure, stabilization of the adhesin is also important. Therefore, constructs are designed to express ETEC subunits stabilized from misfolding and degradation by donor strand complementation. The donor β strand is provided by the major fimbrial subunit. For example, in the case of CfaE, stabilization is provided by the N-terminal region of CfaB. Engineering of dscCfaE by inco...
example 2
CS6 and CS3
[0068]Rabbit model (RITARD) studies suggest the colonization factor CS6 and CS3 has immune-protective potential (Svennerholm, et al., Infect. Immun. 56: 523-528 (1988); Svennerholm, et al., Infect. Immun. 58: 341-346 (1990)). As such, an important technical goal is to reproduce a stabilized CS6 expressing recombinant structure expressing CS6 antigens that maximally elicits antibody responses inhibitory to CS6-directed adhesion.
[0069]Unlike class 5 ETEC fimbriae, the fimbrial structures may function as polyadhesins rather than monadhesins (Zavialov, et al., FEMS Microbiol. Rev. 31: 478-514 (2007)). Extrapolation from related fimbriae, assembly of ETEC CS6 and CS3 may be mediated by a donor strand complementation mediated process through association of a CS6 or CS3 subunit with the N-terminal donor strand region of an adjacent subunit. Additionally, protection against misfolding and proteolytic degradation may also be afforded through donor strand complementation.
[0070]Asso...
example 4
Construction of Multipartite Fusion Constructs
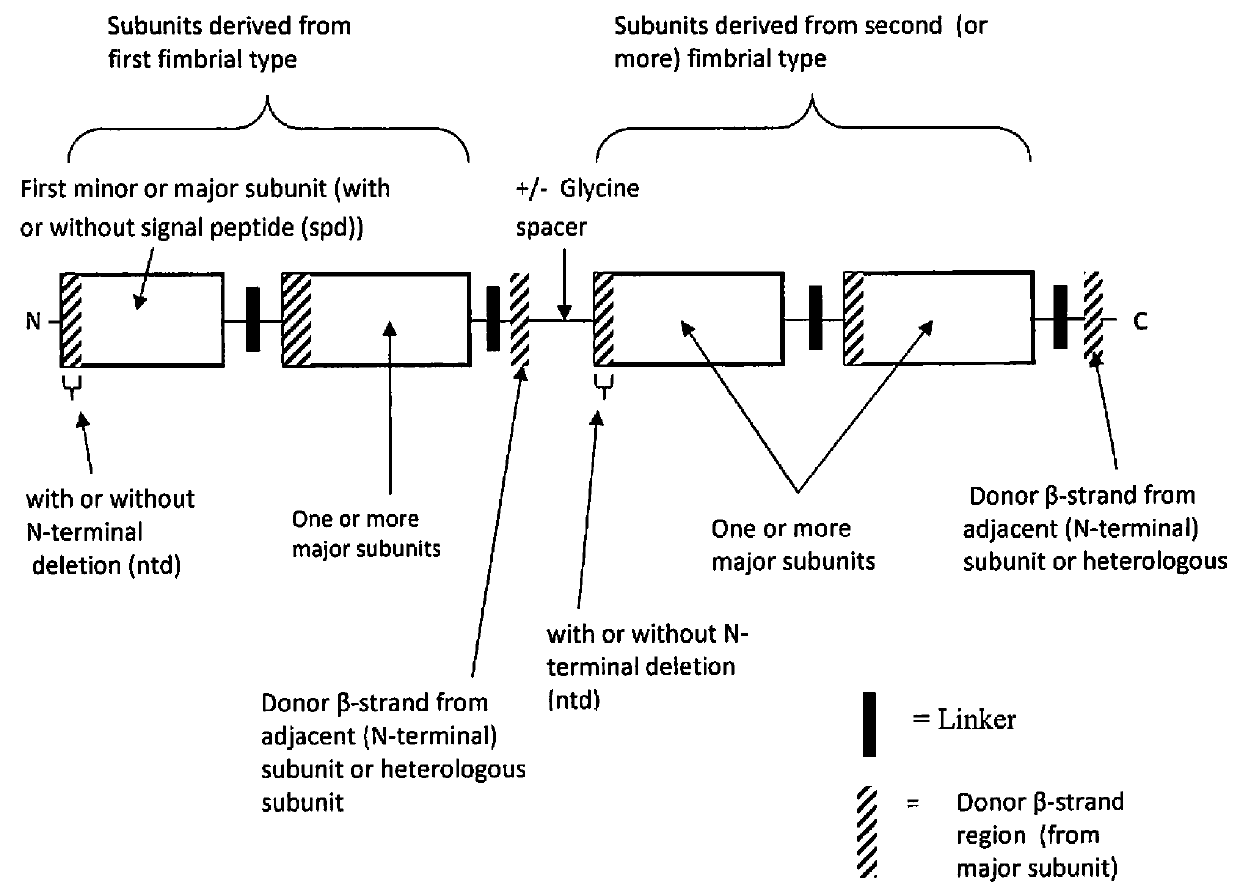
[0096]Immunity to multiple strains of ETEC is important to obtain the greatest extent of anti-ETEC immunity. Toward this goal, recombinant polypeptide constructs were developed comprising two or more subunits derived from different ETEC fimbrial types according to the design illustrated in FIG. 2 to form multipartite fusion constructs. As used, herein, multipartite fusion or multipartite fusion constructs are recombinant polypeptide constructs according to FIG. 2. In this design, different ETEC fimbrial types are defined as fimbrial proteins derived from fimbriae of different strain ETEC types, as listed in Table 6, or deriviates of these polypeptides or DNA sequences. For example, the fimbrial type “CS3” comprises CstH and CstG. The fimbrial type “C56” comprises CssA and CssB. The fimbrial types of Class 5 ETEC include the fimbrial types Class 5a, Class 5b and Class 5c.
[0097]In a preferred embodiment, major and / or minor subunits, derive...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volumes | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| heat-labile | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com