Galvanically functionalized sensors
a functionalized sensor and galvanic technology, applied in the field of galvanically functionalized sensors, can solve the problems of limited number of reducible species in typical in-vivo measurements, high overpotential, and danger of inducing undesirable side reactions, so as to achieve the effect of reducing the number of analyte test strips, and reducing the current of the new sensor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Potential Stability After Partial Oxidation
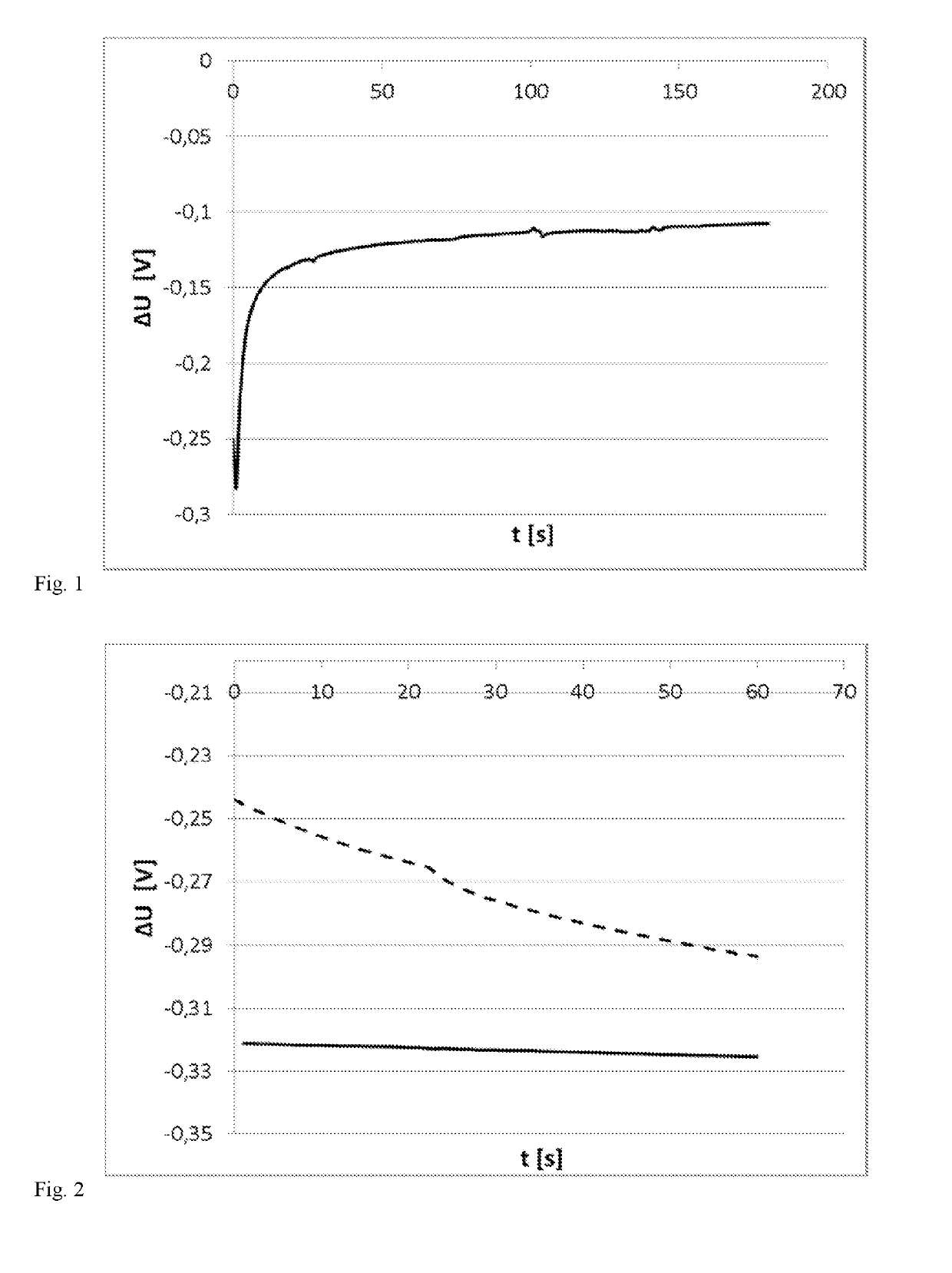
[0115]To test usability as reference electrode, the Ag-coated reference electrode of Example 1 was tested for potential stability. The electrode initially showed the expected reference potential (approx. 275-325 mV vs. manganese dioxide) without oxidation; however, the potential was not stable. After a short (10 seconds) galvanostatic oxidation at 1 μA in a chloride-ion containing solution, the potential against MnO2 was essentially constant. The ratio Ag / AgCl was adjusted via the amount of charge applied. In FIG. 2, 45 mC Ag deposition (Example 1) (dashed line) and only 10 μC oxidation to AgCl (solid line) were used, which reduced potential drift drastically.
example 3
MnO2 Deposition on a Substrate: Working Electrode
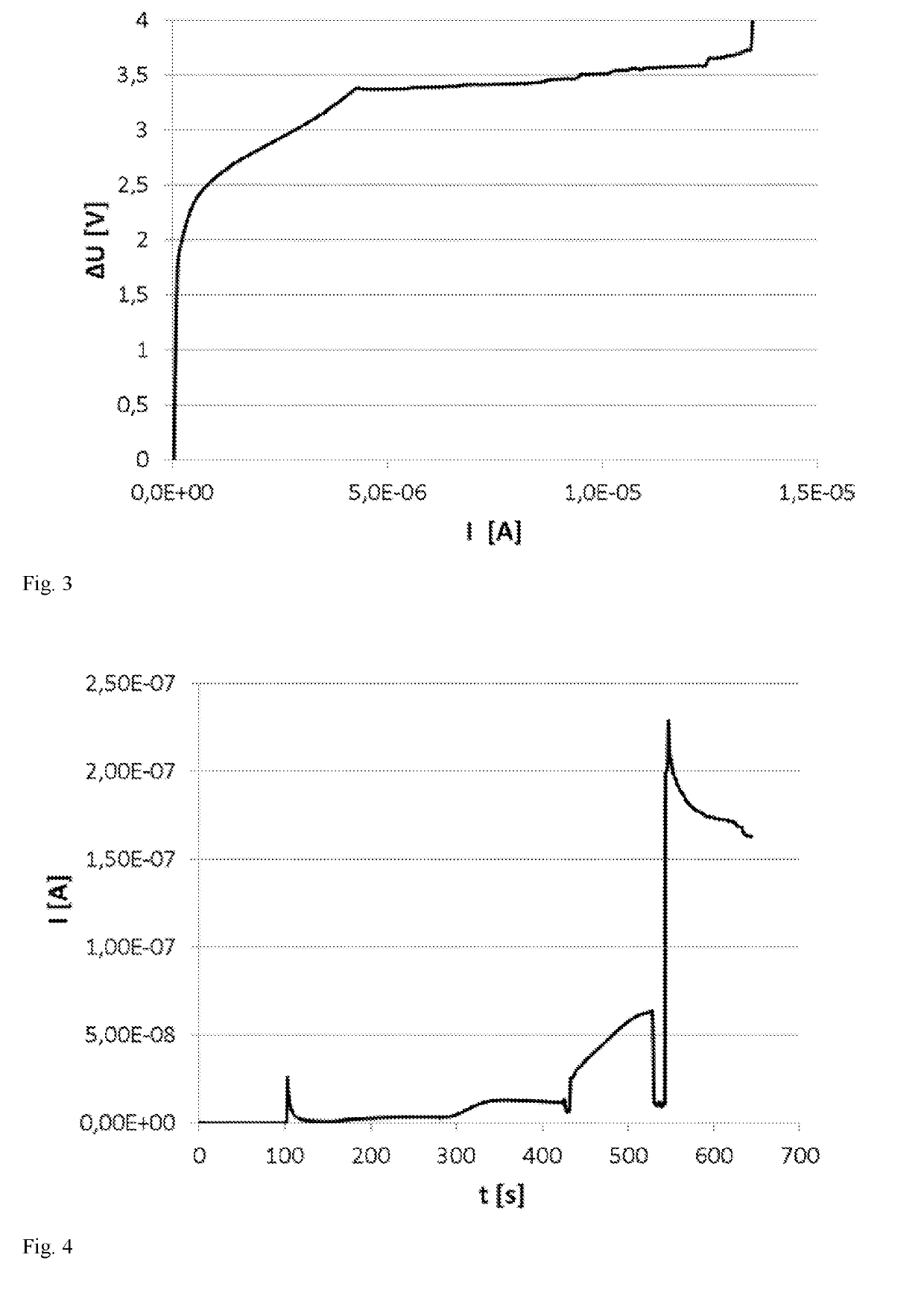
[0116]MnO2 was deposited onto multiple pads of a sensor electrode in a 2-electrode setup (working electrode, reference electrode / counter electrode, sensor reference electrode unused), using a galvanostatic CV from 0 to 15 μA (vs. reference electrode / counter electrode in Mn2+); deposition potential was 3.4 V with a disc voltage reference electrode=counter electrode at 2 V on sensor counter electrode (FIG. 3). Under these conditions, MnO2 was deposited onto all working electrode pads. Accordingly, galvanically depositing MnO2 is possible.
example 4
Functionality Testing (H2O2-Oxidation)
[0117]Functionality of the electrodeposited MnO2 in H2O2-oxidation and functionality of the Ag / AgCl reference electrode were tested in chronoamperometry at 350 mV vs. sensor reference and addition of H2O2. As shown in FIG. 4, the sensor shows an H2O2-dependent signal at 350 mV. Notably, the zero current is very low (approx. 50 pA). Moreover, a running-in of zero current is not observed, since the galvanically deposited electrode does not contain ether peroxides from the paste solvent DEGMBE.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap