Formulations of Terlipressin
a technology of terlipressin and terlipressin, which is applied in the direction of infusion syringes, peptide/protein ingredients, inorganic non-active ingredients, etc., and can solve the problem of side effects in up to 40% of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Acid Formulations
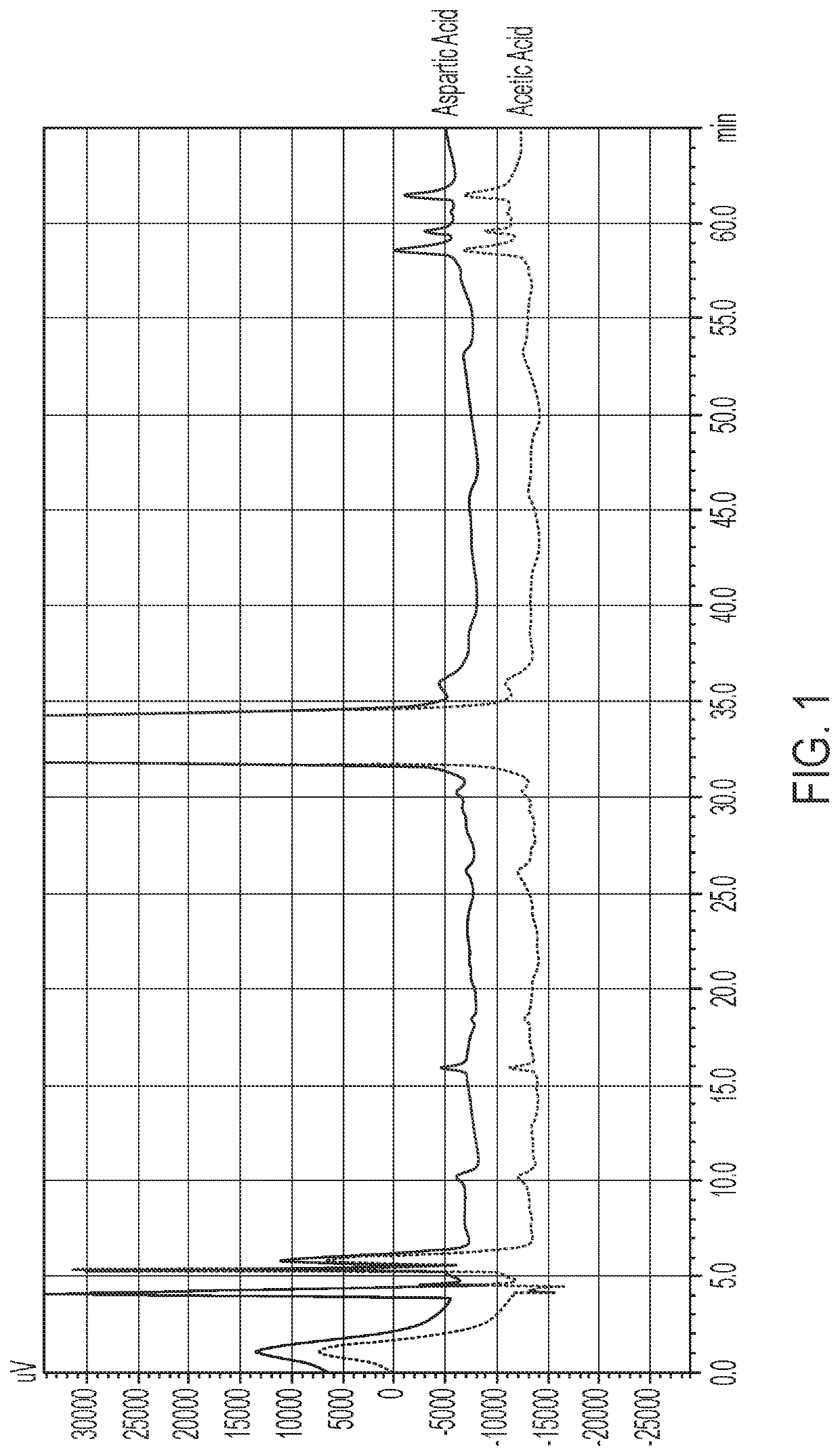
[0091]Terlipressin acetate formulations containing aspartic acid (with and without nitrogen) at a pH of 4.5 are prepared.
[0092]With Nitrogen Protection: Perform all procedures using N2 protection. Sparge water for injection (WFI) prior to addition of materials. Overlay N2 during mix operations, discontinuing only to add solid materials. Keep solution at 20-25 ° C.
[0093]Add WFI to formulation vessel, equivalent to 80% of the final QS volume.
[0094]Add aspartic acid to WFI; rinse aspartic acid weighing vessel with WFI (rinse volume < / =to 3% of final volume). Mix to dissolve
[0095]Adjust pH to 4.0 with 0.1 N NaOH solution.
[0096]Add terlipressin acetate with WFI rinses (rinse volume < / =5% of QS volume). Mix to dissolve.
[0097]Adjust pH to 4.5 with either 0.1 N NaOH or 0.1 N acetic acid solution (expect the pH to be 4.6 after addition of terlipressin acetate to pH 4.0 solution).
[0098]Bring to final volume with low O2 WFI. Readjust pH if required.
[0099]Without Nitrogen Prote...
example 2
id Formulations
[0107]Terlipressin acetate formulations containing acetic acid (with and without nitrogen) at a pH of 4.5 are prepared.
[0108]With Nitrogen Protection: Perform all procedures using N2 protection. Sparge WFI prior to addition of materials. Overlay N2 during mix operations, discontinuing only to add solid materials. Keep solution at 20-25° C.
[0109]Add WFI to formulation vessel, equivalent to 80% of the final QS volume.
[0110]Add acetic acid to WFI; rinse ascetic acid weighing vessel with WFI (rinse volume < / = to 3% of final volume). Mix to dissolve
[0111]Adjust pH to 4.0 with 0.1 N NaOH solution.
[0112]Add terlipressin acetate with WFI rinses (rinse volume < / =5% of QS volume). Mix to dissolve.
[0113]Adjust pH to 4.5 with either 0.1 N NaOH or 0.1 N acetic acid solution (expect the pH to be 4.6 after addition of terlipressin acetate to pH 4.0 solution).
[0114]Bring to final volume with low O2 WFI. Readjust pH if required.
[0115]Without Nitrogen Protection: Keep solution at 20-25...
example 3
of Terlipressin Formulations
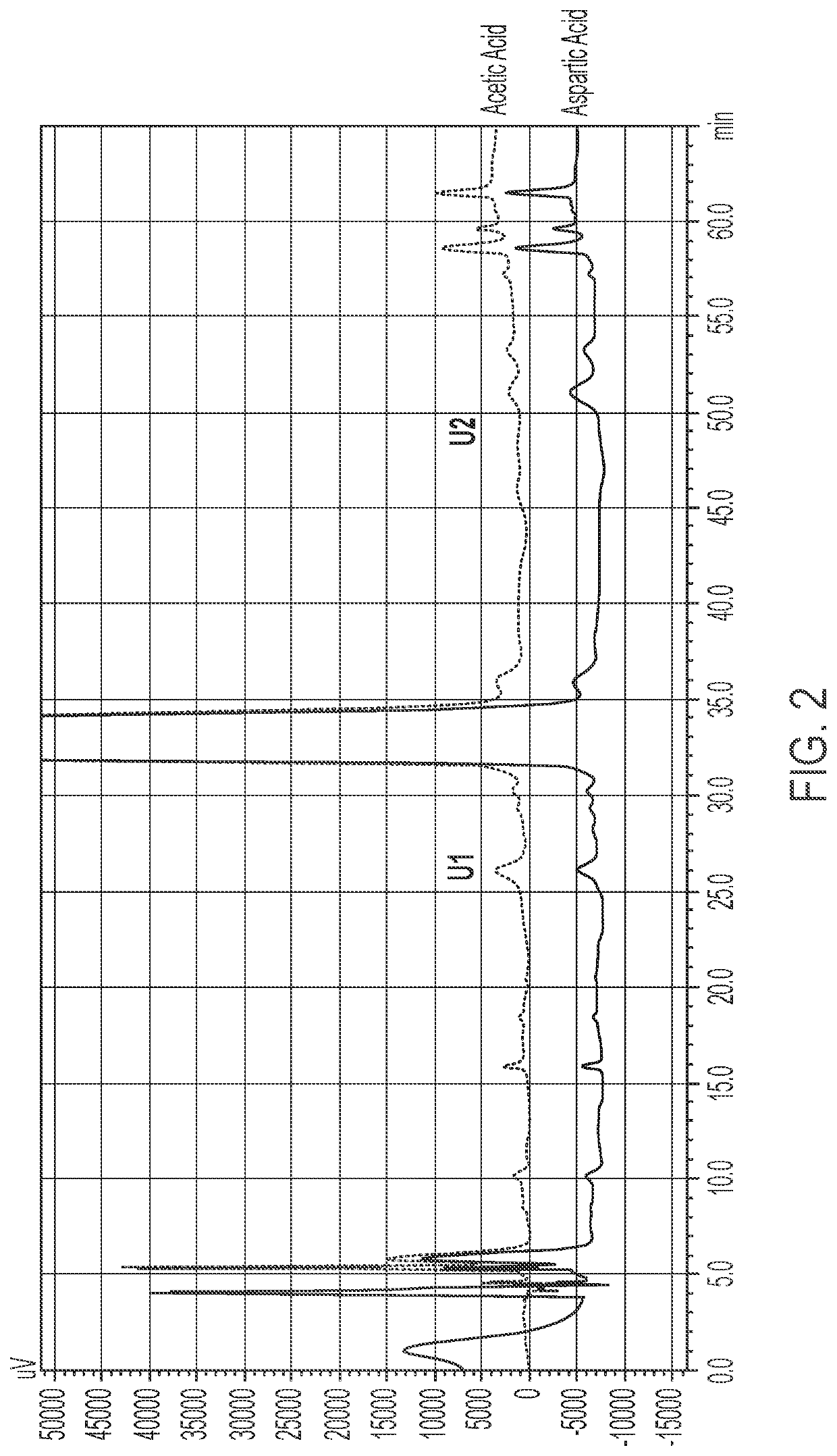
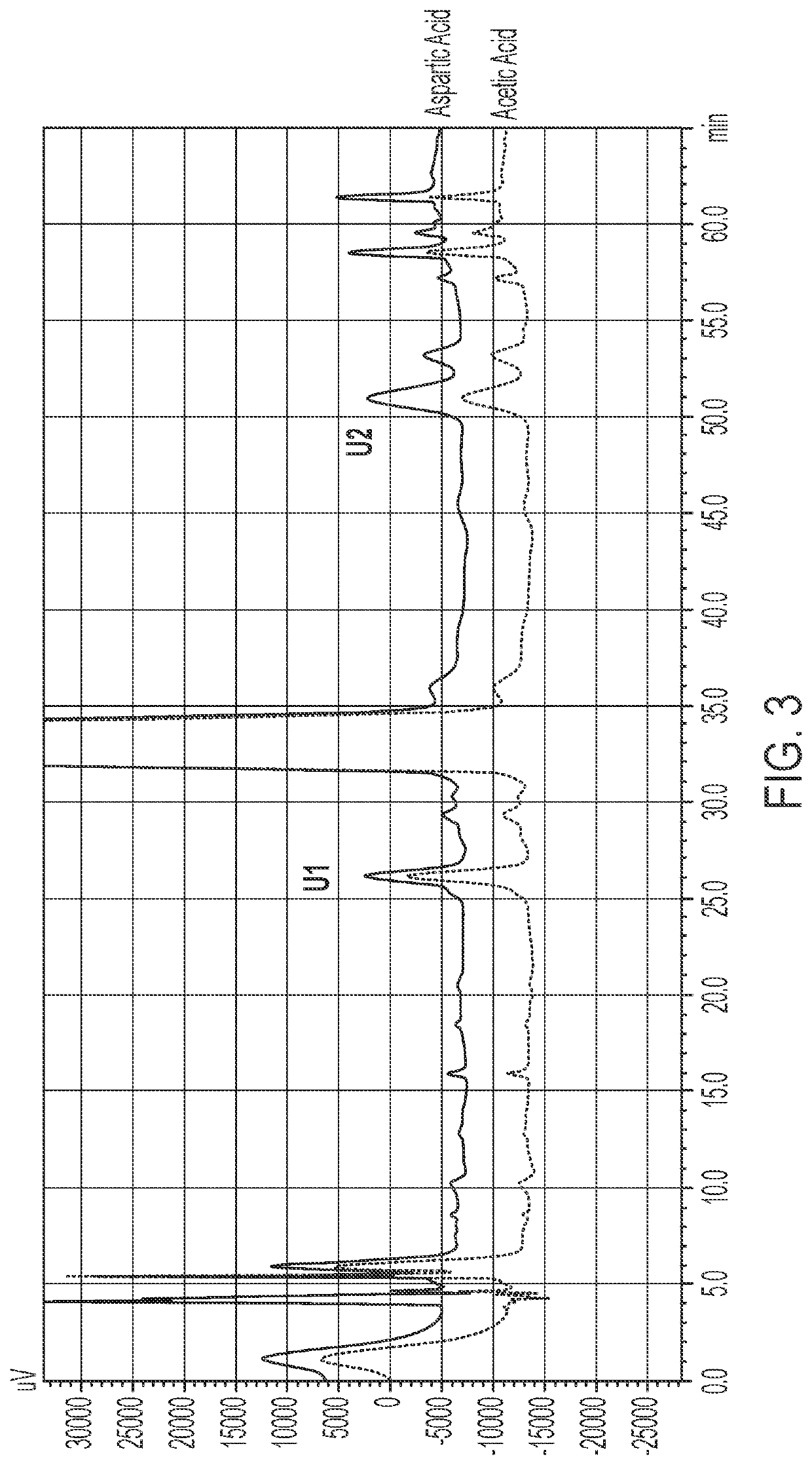
[0123]The impact of aspartic acid formulations with and without nitrogen at pH 4.5 and acetic acid formulations with and without nitrogen at pH 4.5 were assessed at 2° C.-8° C., 25° C. and 40° C. at 3 months and 4 months. The aspartic acid and acetic acid formulations were prepared by methods similar to those described in Examples 1 and 2. The results shown in Tables 6-9 below are shown in terms of % label strength (LS) for the active ingredient (terlipressin acetate), as well as the % change as compared to the day 0 (initial) time point. Table 10 shows a comparison of the %LS data presented in Tables 6-9. Percent label strength was calculated using the following formula: (mg / mL found) / (1.0 mg / mL label strength)×100%.
TABLE 6Aspartic Acid Formulation at pH 4.5 Stability2° C-8° C.25° C.40° C.Time%%%%%%(months)LSchangeLSchangeLSchange096.95—96.95—96.95—396.53−0.4295.87−1.0894.00−2.95498.341.3997.380.4394.30−2.65
TABLE 7Aspartic Acid Formulation with Nitrogen ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com